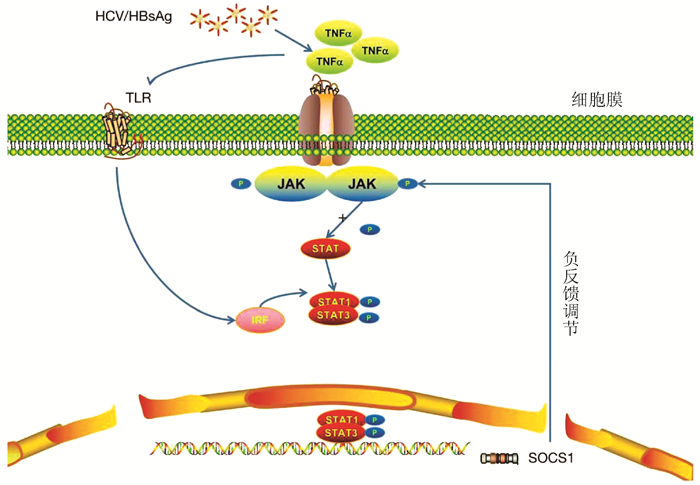
细胞因子信号转导抑制因子1在肝脏炎症性疾病发生发展中的作用机制
DOI: 10.3969/j.issn.1001-5256.2021.04.055
利益冲突声明:所有作者均声明不存在利益冲突。
作者贡献声明:吴霞负责资料分析,撰写论文;朱晓宁、张玉蓉、尹玥、彭孟云、郑丁参与收集数据,修改论文;汪静负责拟定写作思路,指导撰写文章并最后定稿。
Mechanism of action of suppressor of cytokine signaling 1 in the development and progression of liver inflammatory diseases
-
摘要: 肝脏炎症性疾病的发生与自身免疫和炎症反应有关,细胞因子信号转导抑制因子1(SOCS1)作为一种细胞信号的负反馈调节因子,其在炎症性疾病的发生发展中起到关键作用。介绍了SOCS1在自身免疫与炎症反应中的作用机制,简述其在肝脏炎症性疾病如病毒性肝炎、非酒精性脂肪性肝炎等疾病发生、发展中的作用。分析表明SOCS1在炎症反应中的异常表达与细胞因子受体、Toll样受体和激素受体信号的调控有关,从而导致炎症性疾病的发生,因此SOCS1作为肝脏炎症性疾病诊断和治疗的辅助手段具有潜在的发展前景。
-
关键词:
- 肝疾病 /
- 细胞因子信号传导抑制蛋白1 /
- 基因表达调控
Abstract: The development of liver inflammatory diseases is associated with autoimmunity and inflammatory response. As a negative feedback regulator of cell signal, suppressor of cytokine signaling 1 (SOCS1) plays a key role in the development and progression of inflammatory diseases. This article mainly introduces the mechanism of action of SOCS1 in autoimmunity and inflammatory response and briefly describes its role in the development and progression of liver inflammatory diseases such as viral hepatitis and nonalcoholic steatohepatitis. The analysis shows that the abnormal expression of SOCS1 in inflammatory response is associated with the regulation of cytokine receptor, Toll-like receptor, and hormone receptor signal, which leads to the development of inflammatory diseases. Therefore, SOCS1 has potential prospects as an auxiliary means for the diagnosis and treatment of liver inflammatory diseases. -
表 1 SOCS1在不同病因肝脏炎症中可能的作用机制
病因分类 病因相关SOCS1调节因子 SOCS1表达 调控信号通路 机制/结局 参考文献 HBV HBcAg18-27蛋白 降低 IFNγ/STAT1 Th1/Th2增加,HBV降低 Tang等[26] HBsAg 增加 TLR IFNα表达增加 Xu等[27] HCV HCV蛋白、IFN 增加 JAK/STAT 清除HCV Singh等[22] 肝脏缺血再灌注 miRNA-155 降低 NF-κB TNFα、IL-6表达增加 Tan等[35] PBC VDR 降低 miRNA-155-SOCS1 miRNA-155表达上调、持续炎症 Yuan等[36] NAFLD 早期生长反应蛋2 增加 JAK2/STAT3/SOCS1 脂肪变性 Kempinska-Podhorodecka等[37] 熊去氧胆酸 降低 NF-κB/STAT3 FFA、TG分泌降低 Chen等[38] 利莫那班 降低 AMPK磷酸化 改善脂肪变性 Chang等[39] 药物性肝损伤 雷公藤内酯醇 增加 Notch信号通路 ALT、AST分泌增加 Wang等[40] 血吸虫肝病 科利拉金 增加 IL-13/STAT6 肉芽肿减小 Du等[41] 肝衰竭 脂多糖/D-半乳糖胺 增加 JAK2/STAT1 抑制TLR4通路 Li等[42] 注:AMPK,AMP依赖的蛋白激酶;FFA,游离脂肪酸;TG,总胆固醇。 -
[1] World Health Organization. Global hepatitis report, 2017[R/OL]. http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/. [2] ZHANG S, WANG F, ZHANG Z. Current advances in the elimination of hepatitis B in China by 2030[J]. Front Med, 2017, 11(4): 490-501. DOI: 10.1007/s11684-017-0598-4. [3] GAO Y, YANG J, SUN F, et al. Prevalence of anti-HCV antibody among the general population in mainland China between 1991 and 2015: A systematic review and meta-analysis[J]. Open Forum Infect Dis, 2019, 6(3): ofz040. DOI: 10.1093/ofid/ofz040. [4] FAN JG. Epidemiology of alcoholic and nonalcoholic fatty liver disease in China[J]. J Gastroenterol Hepatol, 2013, 28 Suppl 1: 11-17. DOI: 10.1111/jgh.12036. [5] STARR R, WILLSON TA, VINEY EM, et al. A family of cytokine-inducible inhibitors of signalling[J]. Nature, 1997, 387(6636): 917-921. DOI: 10.1038/43206. [6] ENDO TA, MASUHARA M, YOKOUCHI M, et al. A new protein containing an SH2 domain that inhibits JAK kinases[J]. Nature, 1997, 387(6636): 921-924. DOI: 10.1038/43213. [7] GISSELBRECHT S. The CIS/SOCS proteins: A family of cytokine-inducible regulators of signaling[J]. Eur Cytokine Netw, 1999, 10(4): 463-470. http://www.ncbi.nlm.nih.gov/pubmed/10586112 [8] CHANDRAKAR P, PARMAR N, DESCOTEAUX A, et al. Differential induction of SOCS isoforms by leishmania donovani impairs macrophage-T cell cross-talk and host defense[J]. J Immunol, 2020, 204(3): 596-610. DOI: 10.4049/jimmunol.1900412. [9] AHMED CM, LARKIN J 3rd, JOHNSON HM. SOCS1 mimetics and antagonists: A complementary approach to positive and negative regulation of immune function[J]. Front Immunol, 2015, 6: 183. DOI: 10.3389/fimmu.2015.00183. [10] DURHAM GA, WILLIAMS J, NASIM MT, et al. Targeting SOCS proteins to control JAK-STAT signalling in disease[J]. Trends Pharmacol Sci, 2019, 40(5): 298-308. DOI: 10.1016/j.tips.2019.03.001. [11] BAETZ A, KOELSCHE C, STREBOVSKY J, et al. Identification of a nuclear localization signal in suppressor of cytokine signaling 1[J]. FASEB J, 2008, 22(12): 4296-4305. DOI: 10.1096/fj.08-116079. [12] ZHANG JG, FARLEY A, NICHOLSON SE, et al. Correction for Zhang et al., The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation[J]. Proc Natl Acad Sci U S A, 2015, 112(22): e2979. DOI: 10.1073/pnas.1507812112. [13] YEGANEH M, GUI Y, KANDHI R, et al. Suppressor of cytokine signaling 1-dependent regulation of the expression and oncogenic functions of p21(CIP1/WAF1) in the liver[J]. Oncogene, 2016, 35(32): 4200-4211. DOI: 10.1038/onc.2015.485. [14] STARR R, WILLSON TA, VINEY EM, et al. A family of cytokine-inducible inhibitors of signalling[J]. Nature, 1997, 387(6636): 917-921. DOI: 10.1038/43206. [15] YING J, QIU X, LU Y, et al. SOCS1 and its potential clinical role in tumor[J]. Pathol Oncol Res, 2019, 25(4): 1295-1301. DOI: 10.1007/s12253-019-00612-5. [16] LIAU N, LAKTYUSHIN A, LUCET IS, et al. The molecular basis of JAK/STAT inhibition by SOCS1[J]. Nat Commun, 2018, 9(1): 1558. DOI: 10.1038/s41467-018-04013-1. [17] SUN R, PARK O, HORIGUCHI N, et al. STAT1 contributes to dsRNA inhibition of liver regeneration after partial hepatectomy in mice[J]. Hepatology, 2006, 44(4): 955-966. DOI: 10.1002/hep.21344. [18] ILANGUMARAN S, BOBBALA D, RAMANATHAN S. SOCS1: Regulator of T cells in autoimmunity and cancer[J]. Curr Top Microbiol Immunol, 2017, 410: 159-189. DOI: 10.1007/82_2017_63. [19] ZHAO ZX, CAI QX, PENG XM, et al. Expression of SOCS-1 in the liver tissues of chronic hepatitis B and its clinical significance[J]. World J Gastroenterol, 2008, 14(4): 607-611. DOI: 10.3748/wjg.14.607. [20] WAHID B, RAFIQUE S, SALEEM K, et al. An increase in expression of SOCS1 gene with increase in hepatitis C virus viral load[J]. J Interferon Cytokine Res, 2018, 38(3): 122-128. DOI: 10.1089/jir.2017.0129. [21] USHIKI T, HUNTINGTON ND, GLASER SP, et al. Rapid inflammation in mice lacking both SOCS1 and SOCS3 in hematopoietic cells[J]. PLoS One, 2016, 11(9): e0162111. DOI: 10.1371/journal.pone.0162111. [22] SINGH P, DASS J FP. A biomolecular network driven proteinic interaction in HCV clearance[J]. Cell Biochem Biophys, 2018, 76(1-2): 161-172. DOI: 10.1007/s12013-017-0837-y. [23] WAHID B. Role of SOCS1 gene in chronic hepatitis C virus patients: A mini-review[J]. J Interferon Cytokine Res, 2020, 40(9): 454-459. DOI: 10.1089/jir.2018.0109. [24] WANG W, BIAN H, LI F, et al. HBeAg induces the expression of macrophage miR-155 to accelerate liver injury via promoting production of inflammatory cytokines[J]. Cell Mol Life Sci, 2018, 75(14): 2627-2641. DOI: 10.1007/s00018-018-2753-8. [25] LEBOSSÉ F, TESTONI B, FRESQUET J, et al. Intrahepatic innate immune response pathways are downregulated in untreated chronic hepatitis B[J]. J Hepatol, 2017, 66(5): 897-909. DOI: 10.1016/j.jhep.2016.12.024. [26] TANG Y, CHEN X, ZHANG Y, et al. Fusion protein of tapasin and hepatitis B core antigen 18-27 enhances T helper cell type 1/2 cytokine ratio and antiviral immunity by inhibiting suppressors of cytokine signaling family members 1/3 in hepatitis B virus transgenic mice[J]. Mol Med Rep, 2014, 9(4): 1171-1178. DOI: 10.3892/mmr.2014.1947. [27] XU Y, HU Y, SHI B, et al. HBsAg inhibits TLR9-mediated activation and IFN-alpha production in plasmacytoid dendritic cells[J]. Mol Immunol, 2009, 46(13): 2640-2646. DOI: 10.1016/j.molimm.2009.04.031. [28] YOUSSEF SS, HAMDY NM. SOCS1 and pattern recognition receptors: TLR9 and RIG-I; novel haplotype associations in Egyptian fibrotic/cirrhotic patients with HCV genotype 4[J]. Arch Virol, 2017, 162(11): 3347-3354. DOI: 10.1007/s00705-017-3498-7. [29] ESLAM M, VALENTI L, ROMEO S. Genetics and epigenetics of NAFLD and NASH: Clinical impact[J]. J Hepatol, 2018, 68(2): 268-279. DOI: 10.1016/j.jhep.2017.09.003. [30] KEMPINSKA-PODHORODECKA A, WUNSCH E, MILKIEWICZ P, et al. The association between SOCS1-1656G > A polymorphism, insulin resistance and obesity in nonalcoholic fatty liver disease (NAFLD) patients[J]. J Clin Med, 2019, 8(11): 1912. DOI: 10.3390/jcm8111912. [31] YANG G, BADEANLOU L, BIELAWSKI J, et al. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome[J]. Am J Physiol Endocrinol Metab, 2009, 297(1): e211-e224. DOI: 10.1152/ajpendo.91014.2008. [32] SUN Q, XIANG RL, YANG YL, et al. Suppressor of cytokine signaling 1 protects rat pancreatic islets from cytokine-induced apoptosis through Janus kinase/signal transducers and activators of transcription pathway[J]. Chin Med J (Engl), 2013, 126(21): 4048-4053. http://www.ncbi.nlm.nih.gov/pubmed/24229672 [33] LIANG YB, TANG H, CHEN ZB, et al. Downregulated SOCS1 expression activates the JAK1/STAT1 pathway and promotes polarization of macrophages into M1 type[J]. Mol Med Rep, 2017, 16(5): 6405-6411. DOI: 10.3892/mmr.2017.7384. [34] NORKINA O, DOLGANIUC A, CATALANO D, et al. Acute alcohol intake induces SOCS1 and SOCS3 and inhibits cytokine-induced STAT1 and STAT3 signaling in human monocytes[J]. Alcohol Clin Exp Res, 2008, 32(9): 1565-1573. DOI: 10.1111/j.1530-0277.2008.00726.x. [35] TAN L, JIANG W, LU A, et al. miR-155 aggravates liver ischemia/reperfusion injury by suppressing SOCS1 in mice[J]. Transplant Proc, 2018, 50(10): 3831-3839. DOI: 10.1016/j.transproceed.2018.08.060. [36] YUAN FH, CHEN YL, ZHAO Y, et al. microRNA-30a inhibits the liver cell proliferation and promotes cell apoptosis through the JAK/STAT signaling pathway by targeting SOCS-1 in rats with sepsis[J]. J Cell Physiol, 2019, 234(10): 17839-17853. DOI: 10.1002/jcp.28410. [37] KEMPINSKA-PODHORODECKA A, MILKIEWICZ M, WASIK U, et al. Decreased expression of vitamin D receptor affects an immune response in primary biliary cholangitis via the VDR-miRNA155-SOCS1 pathway[J]. Int J Mol Sci, 2017, 18(2): 289. DOI: 10.3390/ijms18020289. [38] CHEN YS, LIU HM, LEE TY. Ursodeoxycholic acid regulates hepatic energy homeostasis and white adipose tissue macrophages polarization in leptin-deficiency obese mice[J]. Cells, 2019, 8(3): 253. DOI: 10.3390/cells8030253. [39] CHANG E, KIM DH, YANG H, et al. CB1 receptor blockade ameliorates hepatic fat infiltration and inflammation and increases Nrf2-AMPK pathway in a rat model of severely uncontrolled diabetes[J]. PLoS One, 2018, 13(10): e0206152. DOI: 10.1371/journal.pone.0206152. [40] WANG X, SUN L, ZHANG L, et al. Effect of adoptive transfer or depletion of regulatory T cells on triptolide-induced liver injury[J]. Front Pharmacol, 2016, 7: 99. DOI: 10.3389/fphar.2016.00099. [41] DU P, MA Q, ZHU ZD, et al. Mechanism of corilagin interference with IL-13/STAT6 signaling pathways in hepatic alternative activation macrophages in schistosomiasis-induced liver fibrosis in mouse model[J]. Eur J Pharmacol, 2016, 793: 119-126. DOI: 10.1016/j.ejphar.2016.11.018. [42] LI SS, YANG M, CHEN YP, et al. Dendritic cells with increased expression of suppressor of cytokine signaling 1(SOCS1) gene ameliorate lipopolysaccharide/d-galactosamine-induced acute liver failure[J]. Mol Immunol, 2018, 101: 10-18. DOI: 10.1016/j.molimm.2018.05.016. -



 PDF下载 ( 2499 KB)
PDF下载 ( 2499 KB)


 下载:
下载: