Influence of interleukin-6 on the expression and function of programmed death-1 in CD8+ T cells from patients with hepatocellular carcinoma
-
摘要:
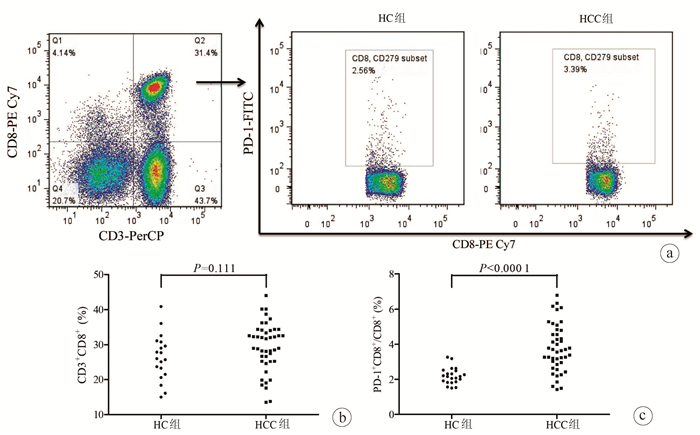
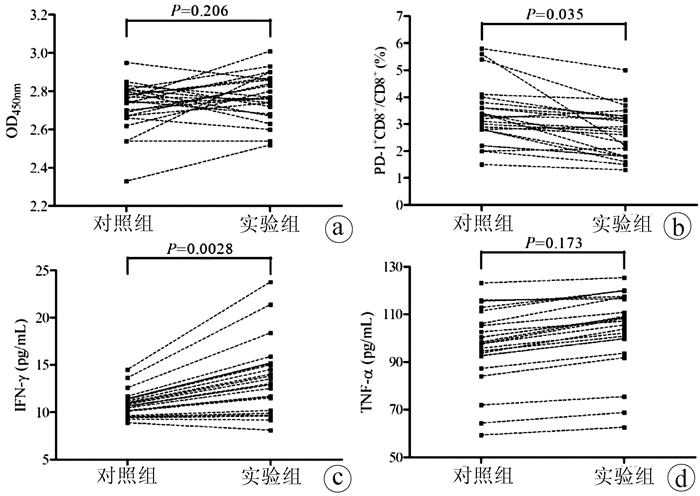
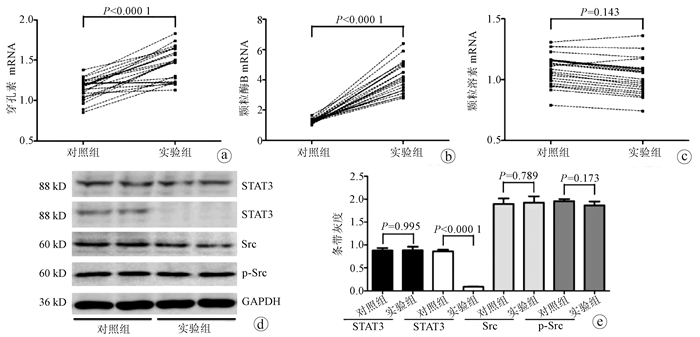
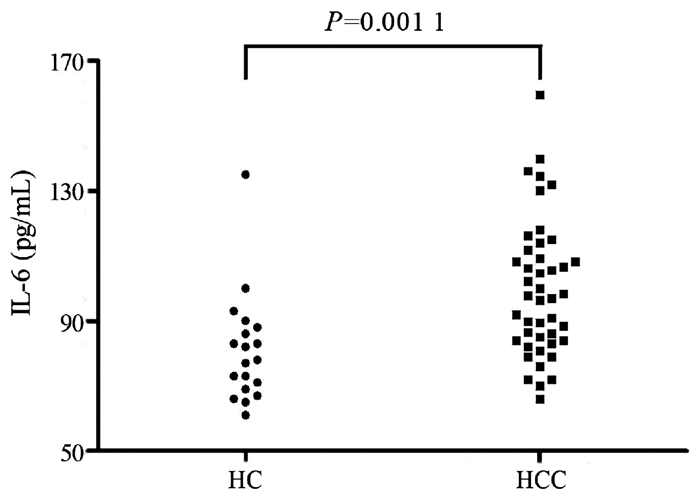
目的 观察血浆IL-6及程序性死亡受体(PD-1)在肝细胞癌(HCC)患者外周血CD8+T淋巴细胞中的表达,评估IL-6对HCC患者CD8+T淋巴细胞中PD-1表达和功能的影响。 方法 纳入2019年1月—2019年9月期间在陕西省人民医院或空军军医大学第二附属医院(第四军医大学唐都医院)就诊的HCC患者44例(HCC组),同时纳入年龄和性别匹配的健康对照者19例(HC组),采集外周血,分离血浆和外周血单个核细胞,分选CD8+T淋巴细胞,ELISA法检测血浆IL-6水平,流式细胞术检测PD-1在CD8+T淋巴细胞中的表达水平。使用IL-6中和抗体刺激分选的CD8+T淋巴细胞24 h,CCK-8法检测细胞增殖,ELISA法检测培养上清IFNγ和TNFα水平,实时定量PCR法检测穿孔素、颗粒酶B和颗粒溶素mRNA相对表达量,Western blot法检测STAT3和Src磷酸化水平。计量资料两组间比较采用t检验或配对t检验;计数资料组间比较采用χ2检验。 结果 HCC组患者血浆IL-6水平较HC组显著升高[(99.67±20.92)pg/mL vs (81.05±16.76)pg/mL,t=3.427,P=0.001 1]。虽然CD3+CD8+T淋巴细胞比例在HCC组和HC组患者之间的差异无统计学意义(P>0.05),但PD-1+CD8+细胞比例在HCC组患者中显著升高(3.79%±1.36% vs 2.20%±0.47%,t=5.335,P<0.000 1)。使用IL-6中和抗体抑制HCC组患者CD8+T淋巴细胞的IL-6虽不影响细胞增殖,但可降低PD-1表达(2.67%±0.91% vs 3.33%±1.12%,t=2.177,P=0.035),增加IFNγ分泌[(13.50±3.82)pg/mL vs (10.82±1.37)pg/mL,t=3.170,P=0.002 8],穿孔素和颗粒酶B mRNA相对表达量亦显著升高(t值分别为6.161、14.140,P值均<0.000 1),同时伴有磷酸化STAT3水平降低(P<0.000 1)。 结论 抗人IL-6中和抗体可能通过增加穿孔素和颗粒酶B水平、增强细胞因子分泌以及抑制PD-1表达增强HCC患者CD8+T淋巴细胞的功能。 -
关键词:
- 癌,肝细胞 /
- CD8阳性T淋巴细胞 /
- 程序性细胞死亡受体1 /
- 白细胞介素6
Abstract:Objective To investigate the influence of interleukin-6(IL-6) on the expression and function of programmed death-1(PD-1) in patients with hepatocellular carcinoma (HCC) by measuring the plasma level of IL-6 and the expression of PD-1 in peripheral blood CD8+ T cells from HCC patients. Methods A total of 44 HCC patients who attended Shaanxi Provincial People's Hospital or The Second Affiliated Hospital of Air Force Medical University & Tangdu Hospital of Fourth Military Medical University from January to September 2019 were enrolled as HCC group, and 19 healthy controls, matched for age and sex, were enrolled as HC group. Peripheral blood was collected, and plasma and peripheral blood mononuclear cells were isolated to separate CD8+ T cells. ELISA was used to measure the plasma level of IL-6, and flow cytometry was used to measure the expression level of PD-1 in CD8+ T cells. The separated CD8+ T cells were stimulated with anti-IL-6 neutralizing antibody for 24 hours; CCK-8 assay was used to measure cell proliferation, ELISA was used to measure the levels of interferon-γ (IFNγ) and tumor necrosis factor-α (TNFα) in supernatant, real-time PCR was used to measure the relative mRNA expression levels of perforin, granzyme B, and granulysin, and Western blot was used to measure the phosphorylation levels of STAT3 and Src. The t-test or the paired t-test was used for comparison of continuous data between two groups, and the chi-square test was used for comparison of categorical data between groups. Results Compared with the HC group, the HCC group had a significant increase in the plasma level of IL-6 (99.67±20.92 pg/mL vs 81.05±16.76 pg/mL, t=3.427, P=0.001 1). There was no significant difference in the percentage of CD3+CD8+ T cells between the HCC group and the HC group, while there was a significant increase in the percentage of PD-1+CD8+ cells in HCC patients (3.79%±1.36% vs 2.20%±0.47%, t=5.335, P < 0.000 1). In the patients with HCC, although anti-IL-6 neutralizing antibody for inhibiting IL-6 in CD8+ T cells did not affect cell proliferation, it downregulated the expression of PD-1 (2.67%±0.91% vs 3.33%±1.12%, t=2.177, P=0.035) and increased the secretion of IFNγ (13.50±3.82 pg/mL vs 10.82±1.37 pg/mL, t=3.170, P=0.002 8), and there were also significant increases in the relative mRNA expression levels of perforin and granzyme B (t=6.161 and 14.140, both P < 0.000 1) and a significant reduction in the level of phosphorylated STAT3 (P < 0.000 1). Conclusion Anti-IL-6 neutralizing antibody can enhance the function of CD8+ T cells in HCC patients possibly by increasing the levels of perforin and granzyme B, improving the secretion of cytokines, and inhibiting the expression of PD-1. -
表 1 实时定量PCR引物序列
基因名称 上游引物(5′-3′) 下游引物(5′-3′) 穿孔素 CGCCTACCTCAGGCTTATCTC CCTCGACAGTCAGGCAGTC 颗粒酶B TGGGGGACCCAGAGATTAAAA TTTCGTCCATAGGAGACAATGC 颗粒溶素 ACTGAAGATGGTGGATAAGCC GCCCTGGGTAACTCTAGACTG GAPDH GCACCGTCAAGGCTGAGAAC TGGTGAAGACGCCAGTGG 表 2 入组志愿者一般资料
资料 HC组
(n=19)HCC组
(n=44)统计值 P值 男/女(例) 11/8 28/16 χ2=0.728 0.537 年龄(岁) 54.2±7.9 58.7±12.1 t=1.486 0.142 AFP(ng/mL) 6.1±1.8 871.2±243.0 t=15.444 <0.000 1 HBV/HCV感染史(例) 0 38 - - -
[1] MCLANE LM, ABDEL-HAKEEM MS, WHERRY EJ. CD8 T cell exhaustion during chronic viral infection and cancer[J]. Annu Rev Immunol, 2019, 37: 457-495. DOI: 10.1146/annurev-immunol-041015-055318. [2] MILLER BC, SEN DR, AL ABOSY R, et al. Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade[J]. Nat Immunol, 2019, 20(3): 326-336. DOI: 10.1038/s41590-019-0312-6. [3] GALLUZZI L, HUMEAU J, BUQUÉ A, et al. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors[J]. Nat Rev Clin Oncol, 2020, 17(12): 725-741. DOI: 10.1038/s41571-020-0413-z. [4] DOLLADILLE C, EDERHY S, SASSIER M, et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer[J]. JAMA Oncol, 2020, 6(6): 865-871. DOI: 10.1001/jamaoncol.2020.0726. [5] ERIKSSON E, MILENOVA I, WENTHE J, et al. IL-6 signaling blockade during CD40-mediated immune activation favors antitumor factors by reducing TGF-β, collagen type I, and PD-L1/PD-1[J]. J Immunol, 2019, 202(3): 787-798. DOI: 10.4049/jimmunol.1800717. [6] LOKAU J, SCHOEDER V, HAYBAECK J, et al. Jak-stat signaling induced by interleukin-6 family cytokines in hepatocellular carcinoma[J]. Cancers (Basel), 2019, 11(11): 1704. DOI: 10.3390/cancers11111704. [7] Bureau of Medical Administrationnational Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)[J]. J Clin Hepatol, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007.中华人民共和国国家卫生健康委员会医政医管局. 原发性肝癌诊疗规范(2019年版)[J]. 临床肝胆病杂志, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007. [8] HOU H, KANG Y, ZENG Y, et al. Interleukin-7 augments CD8(+) T cells function and promotes viral clearance in chronic hepatitis C virus infection[J]. Cytokine, 2018, 102: 26-33. DOI: 10.1016/j.cyto.2017.12.014. [9] YANG YM, KIM SY, SEKI E. Inflammation and liver cancer: Molecular mechanisms and therapeutic targets[J]. Semin Liver Dis, 2019, 39(1): 26-42. DOI: 10.1055/s-0038-1676806. [10] BOMMARITO D, HALL C, TAAMS LS, et al. Inflammatory cytokines compromise programmed cell death-1 (PD-1)-mediated T cell suppression in inflammatory arthritis through up-regulation of soluble PD-1[J]. Clin Exp Immunol, 2017, 188(3): 455-466. DOI: 10.1111/cei.12949. [11] YIN Z, MA T, LIN Y, et al. IL-6/STAT3 pathway intermediates M1/M2 macrophage polarization during the development of hepatocellular carcinoma[J]. J Cell Biochem, 2018, 119(11): 9419-9432. DOI: 10.1002/jcb.27259. [12] BERGMANN J, MVLLER M, BAUMANN N, et al. IL-6 trans-signaling is essential for the development of hepatocellular carcinoma in mice[J]. Hepatology, 2017, 65(1): 89-103. DOI: 10.1002/hep.28874. [13] LI Y, CHEN G, HAN Z, et al. IL-6/STAT3 signaling contributes to sorafenib resistance in hepatocellular carcinoma through targeting cancer stem cells[J]. Onco Targets Ther, 2020, 13: 9721-9730. DOI: 10.2147/OTT.S262089. [14] YANG J, WANG J, LUO J. Decreased IL-6 induces sensitivity of hepatocellular carcinoma cells to sorafenib[J]. Pathol Res Pract, 2019, 215(10): 152565. DOI: 10.1016/j.prp.2019.152565. [15] ALSAAB HO, SAU S, ALZHRANI R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: Mechanism, combinations, and clinical outcome[J]. Front Pharmacol, 2017, 8: 561. DOI: 10.3389/fphar.2017.00561. [16] TSUKAMOTO H, FUJIEDA K, MIYASHITA A, et al. Combined blockade of IL6 and PD-1/PD-L1 signaling abrogates mutual regulation of their immunosuppressive effects in the tumor microenvironment[J]. Cancer Res, 2018, 78(17): 5011-5022. DOI: 10.1158/0008-5472.CAN-18-0118. [17] LIU H, SHEN J, LU K. IL-6 and PD-L1 blockade combination inhibits hepatocellular carcinoma cancer development in mouse model[J]. Biochem Biophys Res Commun, 2017, 486(2): 239-244. DOI: 10.1016/j.bbrc.2017.02.128. [18] ZHANG W, LIU Y, YAN Z, et al. IL-6 promotes PD-L1 expression in monocytes and macrophages by decreasing protein tyrosine phosphatase receptor type O expression in human hepatocellular carcinoma[J]. J Immunother Cancer, 2020, 8(1): e000285. DOI: 10.1136/jitc-2019-000285. [19] WU W, DIETZE KK, GIBBERT K, et al. TLR ligand induced IL-6 counter-regulates the anti-viral CD8(+) T cell response during an acute retrovirus infection[J]. Sci Rep, 2015, 5: 10501. DOI: 10.1038/srep10501. [20] ZHOU X, HOPKINS JW, WANG C, et al. IL-2 and IL-6 cooperate to enhance the generation of influenza-specific CD8 T cells responding to live influenza virus in aged mice and humans[J]. Oncotarget, 2016, 7(26): 39171-39183. DOI: 10.18632/oncotarget.10047. -



 PDF下载 ( 3487 KB)
PDF下载 ( 3487 KB)

 下载:
下载: