转换艾考恩丙替及联合索磷布韦/维帕他韦治疗慢性丙型肝炎初治的HIV/HCV合并感染者的效果及对血脂水平的影响
DOI: 10.3969/j.issn.1001-5256.2022.03.010
Efficacy of switching to co-formulated elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide combined with sofosbuvir/velpatasvir in treatment of previously untreated chronic hepatitis C patients with HIV/HCV co-infection and its influence on blood lipid levels
-
摘要:
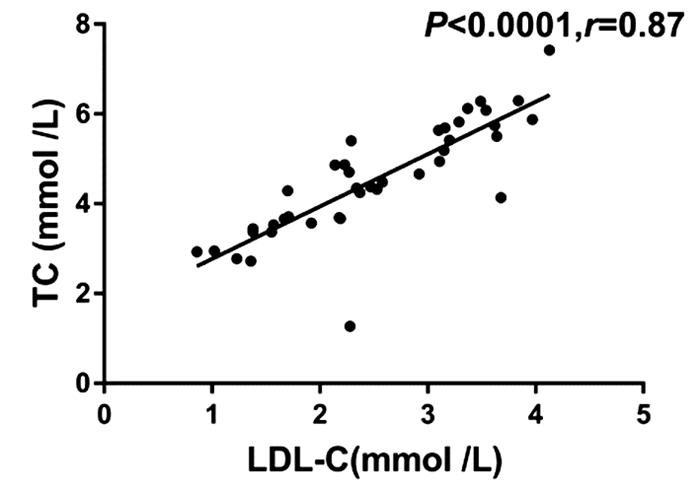
目的 观察转换艾考恩丙替及联合索磷布韦/维帕他韦治疗慢性丙型肝炎初治的HIV/HCV合并感染者的疗效及血脂水平变化。 方法 本研究为前瞻性队列研究。纳入2019年7月—2021年5月于空军军医大学第二附属医院传染科就诊的已接受抗逆转录病毒治疗(ART)并获得HIV持续抑制的、慢性丙型肝炎初治的HIV/HCV合并感染患者10例,将原ART方案转换为艾考恩丙替抗HIV治疗共32周,于转换后第4周开始联合索磷布韦/维帕他韦抗HCV治疗12周,监测10例患者转换艾考恩丙替抗HIV治疗及联合索磷布韦/维帕他韦抗HCV治疗前后体质量、BMI、HCV基因型、AFP、肝脏硬度值、CD4+ T淋巴细胞(简称CD4细胞)数量、CD4+T/CD8+T(简称CD4/CD8)比值、肝肾功能相关指标、血脂相关指标、HIV RNA、HCV RNA、SVR12、SVR24及不良反应发生。计量资料两组间比较采用Mann-Whitney U检验。相关性分析采用Spearman相关性检验。 结果 相较于抗HIV治疗基线(原ART方案),10例患者(HCV基因2a和1b型)转换艾考恩丙替治疗4周后,HIV RNA低于检测下限(20 IU/mL),Alb水平下降(Z=-2.801,P=0.003 7),其他指标均保持稳定,且患者自我报告原ART方案的抗HIV治疗相关不良事件发生情况明显改善。艾考恩丙替联合索磷布韦/维帕他韦4周后的HCV RNA低于检测下限(15 IU/mL),SVR12和SVR24均达100%;相较于抗HCV治疗基线,治疗12周患者ALT(Z=-2.732,P=0.004 8)和AST(Z=-2.501,P=0.010 7)均显著下降;而TC(Z=-2.797,P=0.003 9)及LDL-C(Z=-2.343,P=0.018 5)均较显著回升,且两者呈显著正相关(r=0.87,P<0.001),其他指标均正常。 结论 转换艾考恩丙替及联合索磷布韦/维帕他韦治疗HCV初治的HIV/HCV合并感染者具有良好的疗效、耐受性和安全性,两药的联合既避免了药物之间相互作用,又可获得极高的HCV治愈率,并维持HIV持续病毒学抑制,联合治疗期间TC及LDL-C水平短暂升高,可能反映了HCV感染导致的脂代谢紊乱及该治疗方案的药理作用。 Abstract:Objective To investigate the efficacy of switching to co-formulated elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (E/c/F/TAF) combined with sofosbuvir/velpatasvir (SOF/VEL) in the treatment of previously untreated chronic hepatitis C patients with HIV/HCV co-infection and the changes in blood lipid levels. Methods This prospective cohort study was conducted among 10 previously untreated chronic hepatitis C patients with HIV/HCV co-infection who attended Department of Infectious Diseases in Tangdu Hospital from July 2019 to May 2021 and achieved continuous HIV suppression after antiretroviral treatment (ART). As for anti-HIV therapy, the ART regimen was switched to the E/c/F/TAF regimen for 32 weeks, and for anti-HCV therapy, the SOF/VEL regimen was started since week 4 after switching and lasted for 12 weeks. Related indices were monitored before and after switching to E/c/F/TAF for anti-HCV therapy and SOF/VEL for anti-HCV therapy, including body weight, body mass index, HCV genotype, alpha-fetoprotein, liver stiffness measurement, CD4+T cell count, CD4+T/CD8+T ratio, hepatic and renal function parameters, blood lipids, HIV RNA, HCV RNA, SVR12, SVR24, and adverse reactions. The Mann-Whitney U test was used for comparison of continuous data between two groups, and a Spearman correlation analysis was performed. Results After 4 weeks of treatment with E/c/F/TAF, 10 patients (HCV genotypes 2a and 1b) had HIV RNA below the lower limit of detection (20 IU/ml) and a significant reduction in albumin (Z=-2.801, P=0.003 7), with the other indices remaining stable, and the patients reported significant improvements in the adverse events of anti-HIV therapy with the former ART regimen. After 4 weeks of E/c/F/TAF combined with SOF/VEL, the patients had HCV RNA below the lower limit of detection (15 IU/ml), and both SVR12 and SVR24 reached 100%; after 12 weeks of anti-HCV therapy, there were significant reductions in alanine aminotransferase (Z=-2.732, P=0.004 8) and aspartate aminotransferase (Z=-2.501, P=0.010 7) and significant increases in total cholesterol (TC) (Z=-2.797, P=0.003 9) and low-density lipoprotein cholesterol (LDL-C) (Z=-2.343, P=0.018 5), with a significantly positive correlation between them (r=0.87, P < 0.001), and all the other indices were normal. Conclusion For previously untreated chronic hepatitis C patients with HIV/HCV co-infection, switching to E/c/F/TAF combined with SOF/VEL has good efficacy, tolerability, and safety, and the combination of the two regimens can avoid drug interaction, achieve a high HCV cure rate, and maintain HIV suppression. Transient increases in TC and LDL-C are observed during combination treatment, which suggests dyslipidemia caused by HCV infection and the pharmacological action of this regimen. -
Key words:
- Hepacivirus /
- HIV Infections /
- Antiviral Agents /
- Treatment Outcome
-
表 1 HIV/HCV合并感染患者转换艾考恩丙替及联合索磷布韦/维帕他韦治疗后各项指标变化
指标 转换艾考恩丙替抗HIV治疗基线(n=10) 抗HIV治疗4周(即抗HCV治疗基线,n=10) 抗HCV治疗12周(n=10) PT-12W(n=10) PT-24W(n=10) 体质量(kg) 57.1(51.5~60.9) 59.0(51.7~61.8) 61.5(53.7~65.6) 62.0(54.1~66.8) 62.1(54.9~66.1) BMI(kg/m2) 20.6(19.7~23.6) 21.0(20.5~22.2) 22.2(21.2~25.0) 22.8(21.7~25.5) 22.5(21.2~25.8) CD4细胞数量(个/μL) 579.0(381.0~748.0) 681.0(471.0~1107.0) 685.5(470.8~1107.0) 764.5(654.3~950.5) 656.5(450.8~727.0) CD4/CD8比值 0.7(0.4~1.0) 0.7(0.5~1.1) 0.5(0.5~0.8) 0.9(0.5~1.3) 0.6(0.5~0.9) ALT(U/L) 50.5(37.5~74.3) 60.0(36.5~105.5) 18.0(12.8~24.5)2) 13.0(11.5~17.0) 22.5(18.8~27.5)3) AST(U/L) 36.0(33.8~62.8) 39.5(29.8~63.8) 23.0(18.5~24.0)2) 21.3(17.0~24.5) 25.0(20.0~30.3) TBil(μmol/L) 10.5(7.2~20.5) 10.4(7.8~14.4) 14.0(9.3~16.5) 9.4(6.8~13.0) 8.7(7.9~13.6) Alb(g/L) 46.0(45.1~47.3) 43.7(42.3~45.0)1) 45.9(43.6~48.5) 46.6(42.1~50.7) 47.6(46.3~49.2) eGFR(mL·min-1·1.73 m-2) 115.6(108.2~118.4) 110.8(108.0~123.5) 108.7(103.3~120.5) 110.6(106.9~121.4) 113.9(108.2~117.5) Cr(μmol/L) 51.0(44.5~58.0) 53.0(50.3~59.3) 54.0(46.25~71.5) 58.0(47.5~76.3) 53.5(44.0~63.7) HDL-C(mmol/L) 1.2(0.9~1.3) 1.1(0.9~1.3) 1.4(1.0~1.6) 1.2(1.1~1.6) 1.0(0.8~1.2) LDL-C(mmol/L) 1.9(1.7~2.8) 2.1(1.5~2.3) 3.1(2.1~3.7)2) 3.0(1.5~3.7) 2.3(2.0~3.2) TG(mmol/L) 1.2(0.9~1.4) 1.3(0.9~1.6) 1.3(1.0~2.0) 1.3(0.8~1.6) 1.3(0.7~1.9) TC(mmol/L) 3.8(3.4~4.4) 3.7(3.4~4.4) 5.4(4.4~6.3)2) 4.4(3.4~5.9) 4.7(3.3~5.5) HIV RNA(log10 IU/mL) 未检出 未检出 未检出 未检出 未检出 HCV RNA(log10 IU/mL) 6.1(5.7~6.6) 未检出 未检出 未检出 未检出 不同随访时间的治疗方案 TDF+3TC+EFV(n=6) 艾考恩丙替(n=10) 艾考恩丙替+索磷布 艾考恩丙替(n=10) TDF+3TC+EFV(n=6) TDF+3TC+AZT(n=1) 韦/维帕他韦(n=10) TDF+3TC+AZT(n=1) ABC+3TC+LPV/r(n=1) ABC+3TC+LPV/r(n=1) TDF+3TC+LPV/r(n=2) TDF+3TC+LPV/r(n=2) 注:TDF,富马酸替诺福韦二吡呋酯;3TC,拉米夫定;EFV,依非韦伦;ABC,阿巴卡韦;LPV/r,洛匹那韦/利托那韦;AZT,齐多夫定。与转换艾考恩丙替抗HIV治疗基线比较,1)P<0.05;与抗HCV治疗基线比较,2)P<0.05;与PT-12W比较,3)P<0.05。 -
[1] COLLINS LF, ADEKUNLE RO, CARTWRIGHT EJ. Metabolic syndrome in HIV/HCV co-infected patients[J]. Curr Treat Options Infect Dis, 2019, 11(4): 351-371. DOI: 10.1007/s40506-019-00207-3. [2] NAGGIE S, LUSK S, THOMPSON JW, et al. Metabolomic signature as a predictor of liver disease events in patients with HIV/HCV coinfection[J]. J Infect Dis, 2020, 222(12): 2012-2020. DOI: 10.1093/infdis/jiaa316. [3] KUTI MA, AKINYEMI JO, OGUNBOSI BO, et al. HCV co-infection is associated with metabolic abnormalities among HAART naive HIV-infected persons[J]. Niger J Clin Pract, 2017, 20(7): 799-803. DOI: 10.4103/1119-3077.212444. [4] FELMLEE DJ, HAFIRASSOU ML, LEFEVRE M, et al. Hepatitis C virus, cholesterol and lipoproteins-impact for the viral life cycle and pathogenesis of liver disease[J]. Viruses, 2013, 5(5): 1292-1324. DOI: 10.3390/v5051292. [5] PLATT L, EASTERBROOK P, GOWER E, et al. Prevalence and burden of HCV co-infection in people living with HIV: A global systematic review and meta-analysis[J]. Lancet Infect Dis, 2016, 16(7): 797-808. DOI: 10.1016/S1473-3099(15)00485-5. [6] LO RE V 3rd, KALLAN MJ, TATE JP, et al. Hepatic decompensation in antiretroviral-treated patients co-infected with HIV and hepatitis C virus compared with hepatitis C virus-monoinfected patients: A cohort study[J]. Ann Intern Med, 2014, 160(6): 369-379. DOI: 10.7326/M13-1829. [7] SMITH CJ, RYOM L, WEBER R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D∶A∶D): A multicohort collaboration[J]. Lancet, 2014, 384(9939): 241-248. DOI: 10.1016/S0140-6736(14)60604-8. [8] HINO N, SASAKI R, TAKAHASHI Y, et al. Treatment of hepatitis C virus infection with direct-acting antiviral agents elevates the serum small-dense low-density lipoprotein cholesterol level[J]. Intern Med, 2021, 60(2): 191-199. DOI: 10.2169/internalmedicine.5563-20. [9] BAGELLA P, SQUILLACE N, RICCI E, et al. Lipid profile improvement in virologically suppressed HIV-1-infected patients switched to dolutegravir/abacavir/lamivudine: Data from the SCOLTA project[J]. Infect Drug Resist, 2019, 12: 1385-1391. DOI: 10.2147/IDR.S203813. [10] GRAF C, WELZEL T, BOGDANOU D, et al. Hepatitis C clearance by direct-acting antivirals impacts glucose and lipid homeostasis[J]. J Clin Med, 2020, 9(9): 2702. DOI: 10.3390/jcm9092702. [11] HUNT PW, LEE SA, SIEDNER MJ. Immunologic biomarkers, morbidity, and mortality in treated HIV infection[J]. J Infect Dis, 2016, 214(Suppl 2): s44-s50. DOI: 10.1093/infdis/jiw275. [12] YAN XJ, YANG Y. Research progress on the application of liver transplantation in HIV combined with HCV positive patients[J]. Ogran Transplantation, 2020, 11(6): 677-684. DOI: 10.3969/j.issn.1674-7445.2020.06.005.颜曦婧, 杨扬. 肝移植治疗HIV合并HCV阳性患者的研究进展[J]. 器官移植, 2020, 11(6): 677-684. DOI: 10.3969/j.issn.1674-7445.2020.06.005. [13] SIKAVI C, CHEN PH, LEE AD, et al. Hepatitis C and human immunodeficiency virus coinfection in the era of direct-acting antiviral agents: No longer a difficult-to-treat population[J]. Hepatology, 2018, 67(3): 847-857. DOI: 10.1002/hep.29642. [14] POL S, PARLATI L. Treatment of hepatitis C: The use of the new pangenotypic direct-acting antivirals in "special populations"[J]. Liver Int, 2018, 38(Suppl 1): 28-33. DOI: 10.1111/liv.13626. [15] BERENGUER J, RODRíGUEZ E, MIRALLES P, et al. Sustained virological response to interferon plus ribavirin reduces non-liver-related mortality in patients coinfected with HIV and hepatitis C virus[J]. Clin Infect Dis, 2012, 55(5): 728-736. DOI: 10.1093/cid/cis500. [16] BERENGUER J, RODRÍGUEZ-CASTELLANO E, CARRERO A, et al. Eradication of hepatitis C virus and non-liver-related non-acquired immune deficiency syndrome-related events in human immunodeficiency virus/hepatitis C virus coinfection[J]. Hepatology, 2017, 66(2): 344-356. DOI: 10.1002/hep.29071. [17] ROCKSTROH JK. Optimal therapy of HIV/HCV co-infected patients with direct acting antivirals[J]. Liver Int, 2015, 35(Suppl 1): 51-55. DOI: 10.1111/liv.12721. [18] European Association for the Study of the Liver. EASL clinical practice guidelines: Management of hepatitis C virus infection[J]. J Hepatol, 2011, 55(2): 245-264. DOI: 10.1016/j.jhep.2011.02.023. [19] SCHLABE S, ROCKSTROH JK. Advances in the treatment of HIV/HCV coinfection in adults[J]. Expert Opin Pharmacother, 2018, 19(1): 49-64. DOI: 10.1080/14656566.2017.1419185. [20] AIDS and Hepatitis C Professional Group, Society of Infectious Diseases, Chinese Medical Association; Chinese Center for Disease Control and Prevention. Chinese guidelines for diagnosis and treatment of HIV/AIDS (2018)[J]. Chin J Infect Dis, 2018, 36(12): 705-724. DOI: 10.3760/cma.j.issn.1000-6680.2018.12.001.中华医学会感染病学分会艾滋病丙型肝炎学组, 中国疾病预防与控制中心. 中国艾滋病诊疗指南(2018版)[J]. 中华传染病杂志, 2018, 36(12): 705-724. DOI: 10.3760/cma.j.issn.1000-6680.2018.12.001. [21] MA CT, WANG Q, ZHANG YK, et al. Analysis on the composition of free antiviral drugs for AIDS treatment in China from 2009 to 2019[J]. China Med Herald, 2021, 18(17): 157-160, 168, 198. https://www.cnki.com.cn/Article/CJFDTOTAL-YYCY202117038.htm马春涛, 王强, 张煜昆, 等. 2009—2019年我国艾滋病免费抗病毒治疗药品构成分析[J]. 中国医药导报, 2021, 18(17): 157-160, 168, 198. https://www.cnki.com.cn/Article/CJFDTOTAL-YYCY202117038.htm [22] HEZODE C, REAU N, SVAROVSKAIA ES, et al. Resistance analysis in patients with genotype 1-6 HCV infection treated with sofosbuvir/velpatasvir in the phase Ⅲ studies[J]. J Hepatol, 2018, 68(5): 895-903. DOI: 10.1016/j.jhep.2017.11.032. [23] MILLER MM. Sofosbuvir-velpatasvir: A single-tablet treatment for hepatitis C infection of all genotypes[J]. Am J Health Syst Pharm, 2017, 74(14): 1045-1052. DOI: 10.2146/ajhp60632. [24] ASSELAH T, SHAFRAN SD, BOURGEOIS S, et al. Deferred treatment with a fixed-dose combination of sofosbuvir-velpatasvir for chronic hepatitis C virus genotype 1, 2, 4 and 6 infection[J]. J Viral Hepat, 2019, 26(10): 1229-1232. DOI: 10.1111/jvh.13159. [25] GODINHO R, BUGNON S, GRACIN T, et al. Severe rhabdomyolysis-induced acute kidney injury following concomitant use of Genvoya®(EVG/COBI/FTC/TAF) and simvastatin; a case report[J]. BMC Nephrol, 2019, 20(1): 69. DOI: 10.1186/s12882-019-1257-6. [26] POPESCU CI, RIVA L, VLAICU O, et al. Hepatitis C virus life cycle and lipid metabolism[J]. Biology (Basel), 2014, 3(4): 892-921. DOI: 10.3390/biology3040892. [27] INOUE T, GOTO T, IIO E, et al. Changes in serum lipid profiles caused by three regimens of interferon-free direct-acting antivirals for patients infected with hepatitis C virus[J]. Hepatol Res, 2018, 48(3): e203-e212. DOI: 10.1111/hepr.12970. [28] GONZÁLEZ-ALDACO K, TORRES-REYES LA, OJEDA-GRANADOS C, et al. Immunometabolic effect of cholesterol in hepatitis C infection: Implications in clinical management and antiviral therapy[J]. Ann Hepatol, 2018, 17(6): 908-919. DOI: 10.5604/01.3001.0012.7191. [29] BROWN MS, GOLDSTEIN JL. A receptor-mediated pathway for cholesterol homeostasis[J]. Science, 1986, 232(4746): 34-47. DOI: 10.1126/science.3513311. [30] MEISSNER EG, LEE YJ, OSINUSI A, et al. Effect of sofosbuvir and ribavirin treatment on peripheral and hepatic lipid metabolism in chronic hepatitis C virus, genotype 1-infected patients[J]. Hepatology, 2015, 61(3): 790-801. DOI: 10.1002/hep.27424. [31] BERG T, ANDREONE P, POL S, et al. Low-density lipoprotein and other predictors of response with telaprevir-based therapy in treatment-experienced HCV genotype 1 patients: REALIZE study[J]. Liver Int, 2015, 35(2): 448-454. DOI: 10.1111/liv.12703. [32] LU L, WANG M, XIA W, et al. Migration patterns of hepatitis C virus in China characterized for five major subtypes based on samples from 411 volunteer blood donors from 17 provinces and municipalities[J]. J Virol, 2014, 88(13): 7120-7129. DOI: 10.1128/JVI.00414-14. [33] NIE B, ZHANG KJ, LIU JB, et al. Prevalence of hepatitis C virus genotype in China patients: A systematic review and Meta-analysis[J]. Lab Med Clin, 2016, 13(20): 2876-2881. https://www.cnki.com.cn/Article/CJFDTOTAL-JYYL201620014.htm聂滨, 张开炯, 刘靳波, 等. 中国丙型肝炎病毒基因型分布回顾及Meta分析[J]. 检验医学与临床, 2016, 13(20): 2876-2881. https://www.cnki.com.cn/Article/CJFDTOTAL-JYYL201620014.htm -



 PDF下载 ( 1942 KB)
PDF下载 ( 1942 KB)

 下载:
下载:


