人工智能及机器学习在非酒精性脂肪性肝病中的应用
DOI: 10.3969/j.issn.1001-5256.2022.10.029
利益冲突声明:所有作者均声明不存在利益冲突。
作者贡献声明:冯巩、弥曼、严琴琴、李珊珊负责课题设计,资料分析,撰写论文; 冯巩、王雪莹、郑皓允参与文献检索及相关资料收集; 冯巩、贺娜、弥曼、严琴琴负责拟定写作思路,指导撰写文章并最后定稿。
Application of artificial intelligence and machine learning in non-alcoholic fatty liver research
-
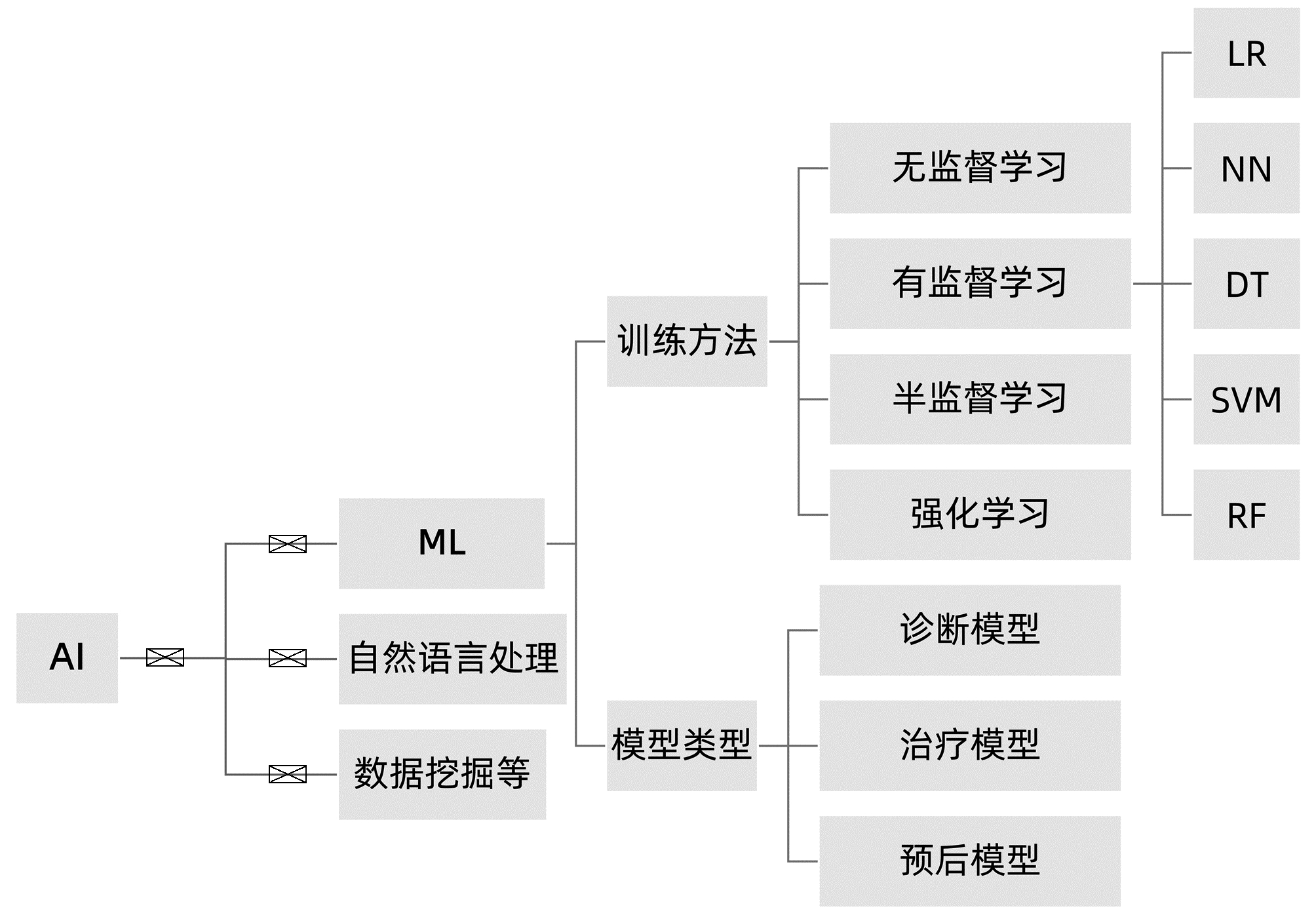
摘要: 非酒精性脂肪性肝病(NAFLD)已成为全球第一大慢性肝病,并与心血管病、肾病发生风险密切相关。目前,NAFLD的诊断和治疗仍然面临诸多挑战,其中,提升诊断效能和优化个体化治疗途径是亟需明确和实现的主要目标。NAFLD严重程度的评估涉及多个临床参数,如何优化非侵入性评估方法是该领域的研究热点。人工智能(AI)在医学领域的应用日益广泛,也为NAFLD的临床诊疗带来新的启示。本文总结了近年来AI及机器学习在NAFLD领域的相关研究成果,阐述了多种相关临床诊断和预后新模型在NAFLD中的应用现状和前景。Abstract: Non-alcoholic fatty liver disease (NAFLD) incidence is rapidly increasing and become the most common chronic liver disease globally. NAFLD also possesses a risk of developing cardiovascular, kidney, and other diseases. To date, NAFLD still faces difficulties in early diagnosis and treatment options. Thus, early detection, prevention, optimally individualized treatment selections, and prediction of prognosis all are the keys in clinical NAFLD control. Although there are assessment tools available for NAFLD severity appraisal using different clinical parameters, it becomes a hot topic of research in the field for how to optimize non-invasive assessment methodologies. Artificial intelligence (AI) and machine learning are increasingly being used in healthcare, especially in assessment and analysis of chronic liver disease, including NAFLD. This review summarized and discussed the most recent progress of AI and machine learning in differential diagnosis of NAFLD and evaluation of NAFLD severity, in order to provide treatment selections, i.e., the novel AI diagnosis models based on the electronic health records and laboratory tests, ultrasound and radiographic imaging, and liver histopathology data. The therapeutic models discussed the personalized lifestyle changes and NAFLD drug development. The NAFLD prognosis model reviewed and predicted how NAFLD-changed liver metabolisms affect prognosis of patients. This review also speculated future prospective research hot spots and development in the filed for how to utilize the existing AI models to distinguish NAFLD and non-alcoholic steatohepatitis (NASH) and assess NAFLD fibrosis status.
-
[1] ESLAM M, SANYAL AJ, GEORGE J, et al. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease[J]. Gastroenterology, 2020, 158(7): 1999-2014. e1. DOI: 10.1053/j.gastro.2019.11.312. [2] FOUAD Y, WAKED I, BOLLIPO S, et al. What's in a name? Renaming 'NAFLD' to 'MAFLD'[J]. Liver Int, 2020, 40(6): 1254-1261. DOI: 10.1111/liv.14478. [3] ZHANG HJ, WANG YY, CHEN C, et al. Cardiovascular and renal burdens of metabolic associated fatty liver disease from serial US national surveys, 1999-2016[J]. Chin Med J (Engl), 2021, 134(13): 1593-1601. DOI: 10.1097/CM9.0000000000001513. [4] BADILLO S, BANFAI B, BIRZELE F, et al. An introduction to machine learning[J]. Clin Pharmacol Ther, 2020, 107(4): 871-885. DOI: 10.1002/cpt.1796. [5] SU TH, WU CH, KAO JH. Artificial intelligence in precision medicine in hepatology[J]. J Gastroenterol Hepatol, 2021, 36(3): 569-580. DOI: 10.1111/jgh.15415. [6] AHN JC, CONNELL A, SIMONETTO DA, et al. Application of artificial intelligence for the diagnosis and treatment of liver diseases[J]. Hepatology, 2020, 73(6): 2546-2563. DOI: 10.1002/hep.31603. [7] CAO W, AN X, CONG L, et al. Application of deep learning in quantitative analysis of 2-dimensional ultrasound imaging of nonalcoholic fatty liver disease[J]. J Ultrasound Med, 2020, 39(1): 51-59. DOI: 10.1002/jum.15070. [8] PERAKAKIS N, POLYZOS SA, YAZDANI A, et al. Non-invasive diagnosis of non-alcoholic steatohepatitis and fibrosis with the use of omics and supervised learning: A proof of concept study[J]. Metabolism, 2019, 101: 154005. DOI: 10.1016/j.metabol.2019.154005. [9] GARCIA-CARRETERO R, VIGIL-MEDINA L, BARQUERO-PEREZ O, et al. Relevant features in nonalcoholic steatohepatitis determined using machine learning for feature selection[J]. Metab Syndr Relat Disord, 2019, 17(9): 444-451. DOI: 10.1089/met.2019.0052. [10] GAWRIEH S, SETHUNATH D, CUMMINGS OW, et al. Automated quantification and architectural pattern detection of hepatic fibrosis in NAFLD[J]. Ann Diagn Pathol, 2020, 47: 151518. DOI: 10.1016/j.anndiagpath.2020.151518. [11] HWANG A, SHI C, ZHU E, et al. Supervised learning reveals circulating biomarker levels diagnostic of hepatocellular carcinoma in a clinically relevant model of non-alcoholic steatohepatitis; An OAD to NASH[J]. PLoS One, 2018, 13(6): e0198937. DOI: 10.1371/journal.pone.0198937. [12] RAMOT Y, ZANDANI G, MADAR Z, et al. Utilization of a deep learning algorithm for microscope-based fatty vacuole quantification in a fatty liver model in mice[J]. Toxicol Pathol, 2020, 48(5): 702-707. DOI: 10.1177/0192623320926478. [13] FORLANO R, MULLISH BH, GIANNAKEAS N, et al. High-throughput, machine learning-based quantification of steatosis, inflammation, ballooning, and fibrosis in biopsies from patients with nonalcoholic fatty liver disease[J]. Clin Gastroenterol Hepatol, 2020, 18(9): 2081-2090. e9. DOI: 10.1016/j.cgh.2019.12.025. [14] DEO RC. Machine learning in medicine[J]. Circulation, 2015, 132(20): 1920-1930. DOI: 10. M16/circuslationaha. 115. 001593. [15] KAVAKIOTIS I, TSAVE O, SALIFOGLOU A, et al. Machine learning and data mining methods in diabetes research[J]. Comput Struct Biotechnol J, 2017, 15: 104-116. DOI: 10.1016/j.csbj.2016.12.005. [16] DINANI AM, KOWDLEY KV, NOUREDDIN M. Application of artificial intelligence for diagnosis and risk stratification in NAFLD and NASH: The state of the art[J]. Hepatology, 2021, 74(4): 2233-2240. DOI: 10.1002/hep.31869. [17] van VLECK TT, CHAN L, COCA SG, et al. Augmented intelligence with natural language processing applied to electronic health records for identifying patients with non-alcoholic fatty liver disease at risk for disease progression[J]. Int J Med Inform, 2019, 129: 334-341. DOI: 10.1016/j.ijmedinf.2019.06.028. [18] WANG K, LU X, ZHOU H, et al. Deep learning radiomics of shear wave elastography significantly improved diagnostic performance for assessing liver fibrosis in chronic hepatitis B: a prospective multicentre study[J]. Gut, 2019, 68(4): 729-741. DOI: 10.1136/gutjnl-2018-316204. [19] TANG A, DESTREMPES F, KAZEMIRAD S, et al. Quantitative ultrasound and machine learning for assessment of steatohepatitis in a rat model[J]. Eur Radiol, 2019, 29(5): 2175-2184. DOI: 10.1007/s00330-018-5915-z. [20] WU CC, YEH WC, HSU WD, et al. Prediction of fatty liver disease using machine learning algorithms[J]. Comput Methods Programs Biomed, 2019, 170: 23-29. DOI: 10.1016/j.cmpb.2018.12.032. [21] HUO Y, TERRY JG, WANG J, et al. Fully automatic liver attenuation estimation combing CNN segmentation and morphological operations[J]. Med Phys, 2019, 46(8): 3508-3519. DOI: 10.1002/mp.13675. [22] GRAFFY PM, SANDFORT V, SUMMERS RM, et al. Auto-mated liver fat quantification at nonenhanced abdominal CT for population-based steatosis assessment[J]. Radiology, 2019, 293(2): 334-342. DOI: 10. 1148/ radiol. 20191 90512. [23] FORLANO R, MULLISH BH, GIANNAKEAS N, et al. High-throughput, machine learning-based quantifica-tion of steatosis, inflammation, ballooning, and fibrosis in biopsies from patients with nonalcoholic fatty liver disease[J]. Clin Gastroen-terol Hepatol, 2020, 18(9): 2081-2090. DOI: 10. 1016/j. cgh. 2019. 12. 025. [24] QU H, MINACAPELLI CD, TAIT C, et al. Training of computational algorithms to predict NAFLD activity score and fibrosis stage from liver histopathology slides[J]. Comput Methods Programs Biomed, 2021, 207: 106153. DOI: 10.1016/j.cmpb.2021.106153. [25] FENG G, ZHENG KI, LI YY, et al. Machine learning algorithm outperforms fibrosis markers in predicting significant fibrosis in biopsy-confirmed NAFLD[J]. J Hepatobiliary Pancreat Sci, 2021, 28(7): 593-603. DOI: 10.1002/jhbp.972. [26] KOLECK TA, DREISBACH C, BOURNE PE, et al. Natural language processing of symptoms documented in free-text narratives of electronic health records: a systematic review[J]. J Am Med Inform Assoc, 2019, 26(4): 364-379. DOI: 10.1093/jamia/ocy173. [27] VANDERBECK S, BOCKHORST J, KLEINER D, et al. Automatic quantification of lobular inflammation and hepatocyte ballooning in nonalcoholic fatty liver disease liver biopsies[J]. Hum Pathol, 2015, 46(5): 767-775. DOI: 10.1016/j.humpath.2015.01.019. [28] VANDERBECK S, BOCKHORST J, KOMOROWSKI R, et al. Automatic classification of white regions in liver biopsies by supervised machine learning[J]. Hum Pathol, 2014, 45(4): 785-792. DOI: 10.1016/j.humpath.2013.11.011. [29] YIP TC, MA AJ, WONG VW, et al. Laboratory parameter-based machine learning model for excluding non-alcoholic fatty liver disease (NAFLD) in the general population[J]. Aliment Pharmacol Ther, 2017, 46(4): 447-456. DOI: 10.1111/apt.14172. [30] PERVEEN S, SHAHBAZ M, KESHAVJEE K, et al. A systematic machine learning based approach for the diagnosis of non-alcoholic fatty liver disease risk and progression[J]. Sci Rep, 2018, 8(1): 2112. DOI: 10.1038/s41598-018-20166-x. [31] BERNÁ G, ROMERO-GOMEZ M. The role of nutrition in non-alcoholic fatty liver disease: Pathophysiology and management[J]. Liver Int, 2020, 40(Suppl 1): 102-108. DOI: 10.1111/liv.14360. [32] ZEEVI D, KOREM T, ZMORA N, et al. Personalized nutrition by prediction of glycemic responses[J]. Cell, 2015, 163(5): 1079-1094. DOI: 10.1016/j.cell.2015.11.001. [33] CALZADILLA-BERTOT L, VILAR-GOMEZ E, WONG VW, et al. ABIDE: An accurate predictive model of liver decompensation in patients with nonalcoholic fatty liver-related cirrhosis[J]. Hepatology, 2021, 73(6): 2238-2250. DOI: 10.1002/hep.31576. [34] SEGAL E. Rich data sets could end costly drug discovery[J]. Nature, 2020, 577(7792): S19. DOI: 10.1038/d41586-020-00200-7. [35] BIANCO C, JAMIALAHMADI O, PELUSI S, et al. Non-invasive stratification of hepatocellular carcinoma risk in non-alcoholic fatty liver using polygenic risk scores[J]. J Hepatol, 2021, 74(4): 775-782. DOI: 10.1016/j.jhep.2020.11.024. [36] YOUNES R, CAVIGLIA GP, GOVAERE O, et al. Long-term outcomes and predictive ability of non-invasive scoring systems in patients with non-alcoholic fatty liver disease[J]. J Hepatol, 2021, 75(4): 786-794. DOI: 10.1016/j.jhep.2021.05.008. [37] PETTA S, SEBASTIANI G, BUGIANESI E, et al. Non-invasive prediction of esophageal varices by stiffness and platelet in non-alcoholic fatty liver disease cirrhosis[J]. J Hepatol, 2018, 69(4): 878-885. DOI: 10.1016/j.jhep.2018.05.019. -



 PDF下载 ( 1869 KB)
PDF下载 ( 1869 KB)

 下载:
下载:


