益生菌对轻微型肝性脑病患者显性肝性脑病发生率影响的Meta分析
DOI: 10.3969/j.issn.1001-5256.2022.11.013
Effect of probiotics in preventing overt hepatic encephalopathy in patients with minimal hepatic encephalopathy: A Meta-analysis
-
摘要:
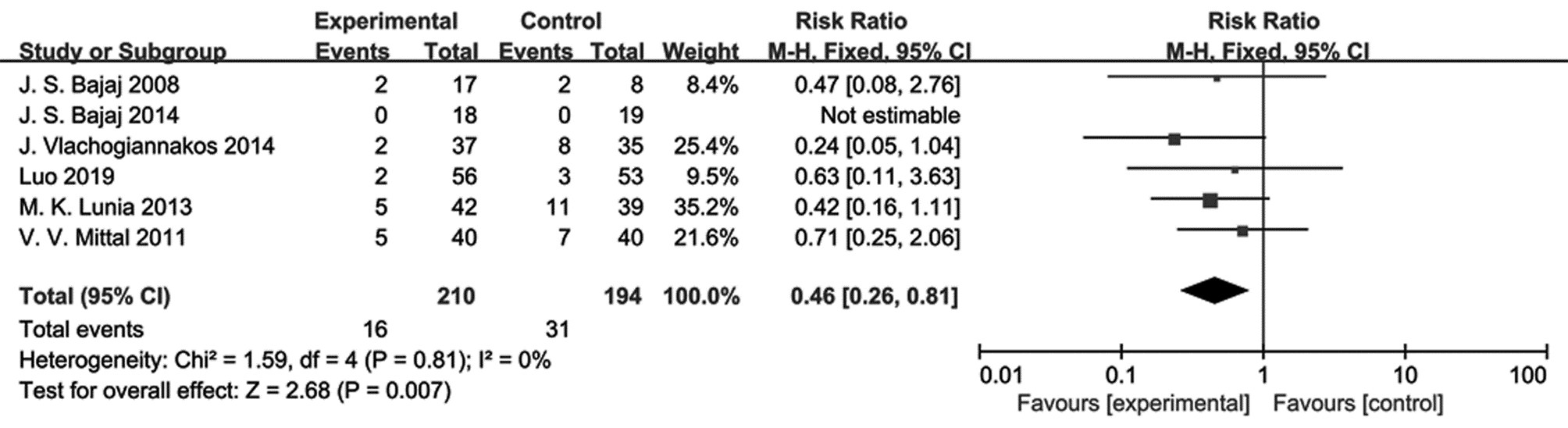
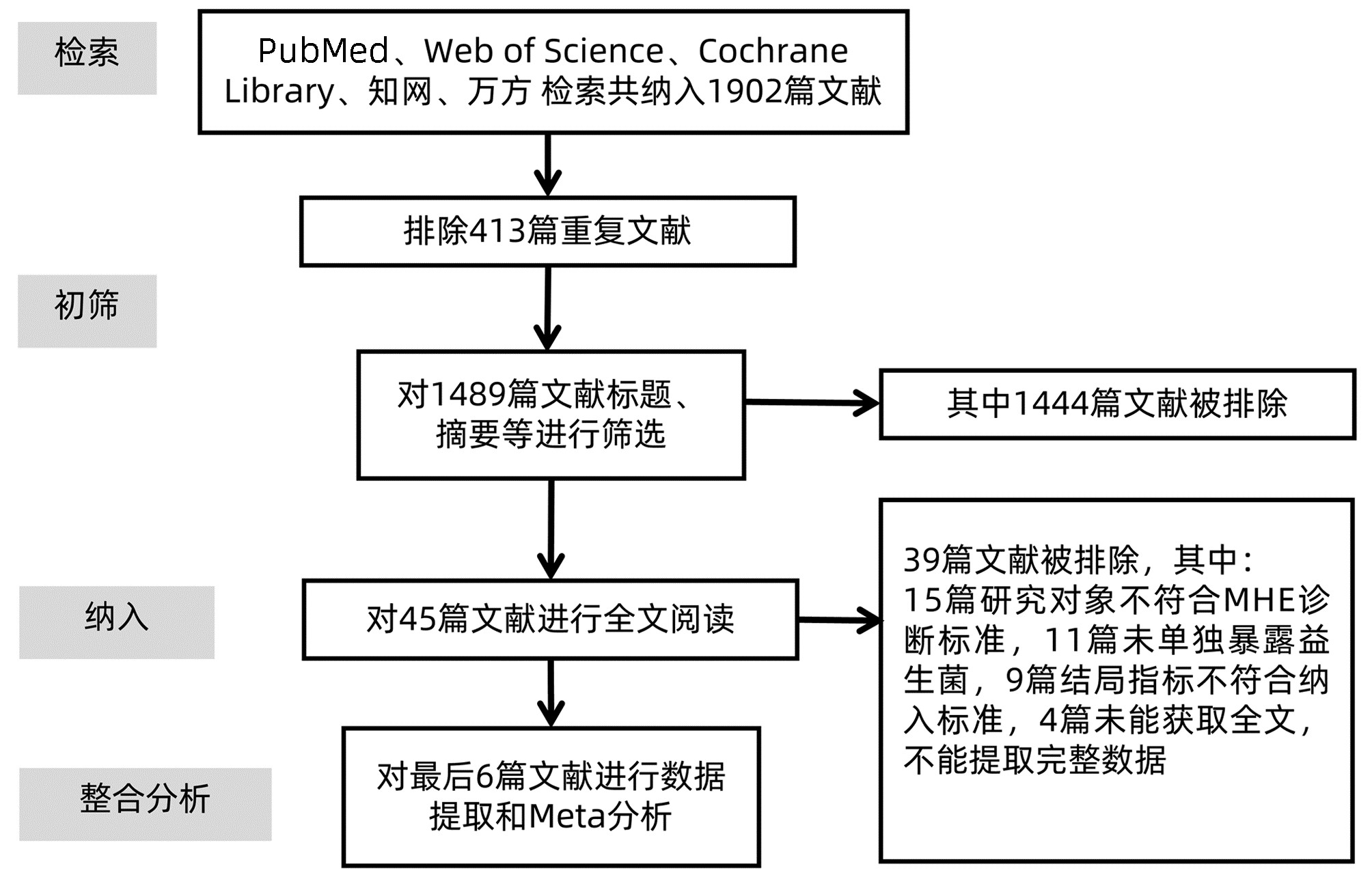
目的 评价使用益生菌干预对轻微型肝性脑病患者显性肝性脑病发生率的影响。 方法 本研究根据PRISMA指南完成,PROSPERO注册号为CRD42022303995。检索PubMed、Web of Science、Cochrane Library、知网、万方数据库,收集建库至2021年11月有关益生菌干预治疗轻微型肝性脑病的研究。用RevMan 5.4进行Meta分析,不能合并的数据采用描述性分析。选择危险比(RR)和95%CI作为汇总指标。 结果 共纳入6篇文献,均为随机对照研究,共404例轻微型肝性脑病患者。结果表明益生菌干预可使轻微型肝性脑病患者显性肝性脑病的发生率降低54%(RR=0.46,95%CI:0.26~0.81,P=0.007),并且能增加轻微型肝性脑病患者疾病的逆转率(RR=4.94,95%CI:2.82~8.66,P<0.000 01)。 结论 益生菌能够降低轻微型肝性脑病患者显性肝性脑病的发生率,增加轻微型肝性脑病的逆转率,对轻微型肝性脑病患者预防显性肝性脑病的发生具有积极意义,为益生菌治疗轻微型肝性脑病提供了新的证据。 Abstract:Objective To review and analyze the effect of probiotics in preventing the overt hepatic encephalopathy (OHE) in patients with minimal hepatic encephalopathy (MHE). Methods Studies about this subject were searched in PubMed, Web of Science, Cochrane Library, Chinese journal full-text database (CNKI), WanFang data knowledge service platform (WanFang Data) from their establishment to November 2021. Meta-analysis was performed using RevMan 5.4. Description analysis was used for data that could not be pooled. The relative risk (RR) and 95% confidence interval (CI) were used to present pooled data. Results Six RCT studies with a total of 404 patients were included in this meta-analysis. The results showed probiotics users had a significant reduction of the OHE incidence, as compared with the controls (RR=0.46, 95%CI: 0.26 - 0.81; P=0.007), but an increase in the reversal MHE rate (RR=4.94, 95%CI: 2.82-8.66; P < 0.000 01). Conclusion This finding demonstrated that probiotics were able to effectively reduce the OHE incidence and improve the reversal MHE rate in the patients with MHE. This study could provide novel evidence for probiotics treatment of MHE. -
Key words:
- Hepatic Encephalopathy /
- Probiotics /
- Meta-Analysis
-
表 1 纳入文献的基本特征
Table 1. Characteristics of the included trials
纳入研究及时间 干预措施 研究时间 结局指标 罗兰[19](2019) 干预组:枯草杆菌二联活菌肠溶胶囊250 mg/粒,2粒,3次/d
对照组:肝硬化常规治疗4周 ①③④⑤⑧⑨ B11 Bajaj等[14](2014) 干预组:乳酸菌(>5×1010 CFU/g)
对照组:安慰剂8周 ①③④⑥⑦⑩ B11 Vlachogiannakos等[18](2014) 干预组:乳酸菌
对照组:安慰剂12周 ①②③④ Lunia等[15](2013) 干预组:益生菌(1.1×1011 CFU/粒) 1粒,3次/d
对照组:无治疗12周 ①②③④ Mittal等[17](2011) 干预组:益生菌(1.1×1011 CFU)次/d
对照组:肝硬化常规治疗12周 ①②③④⑦ Bajaj等[13](2008) 干预组:酸奶12盎司/d
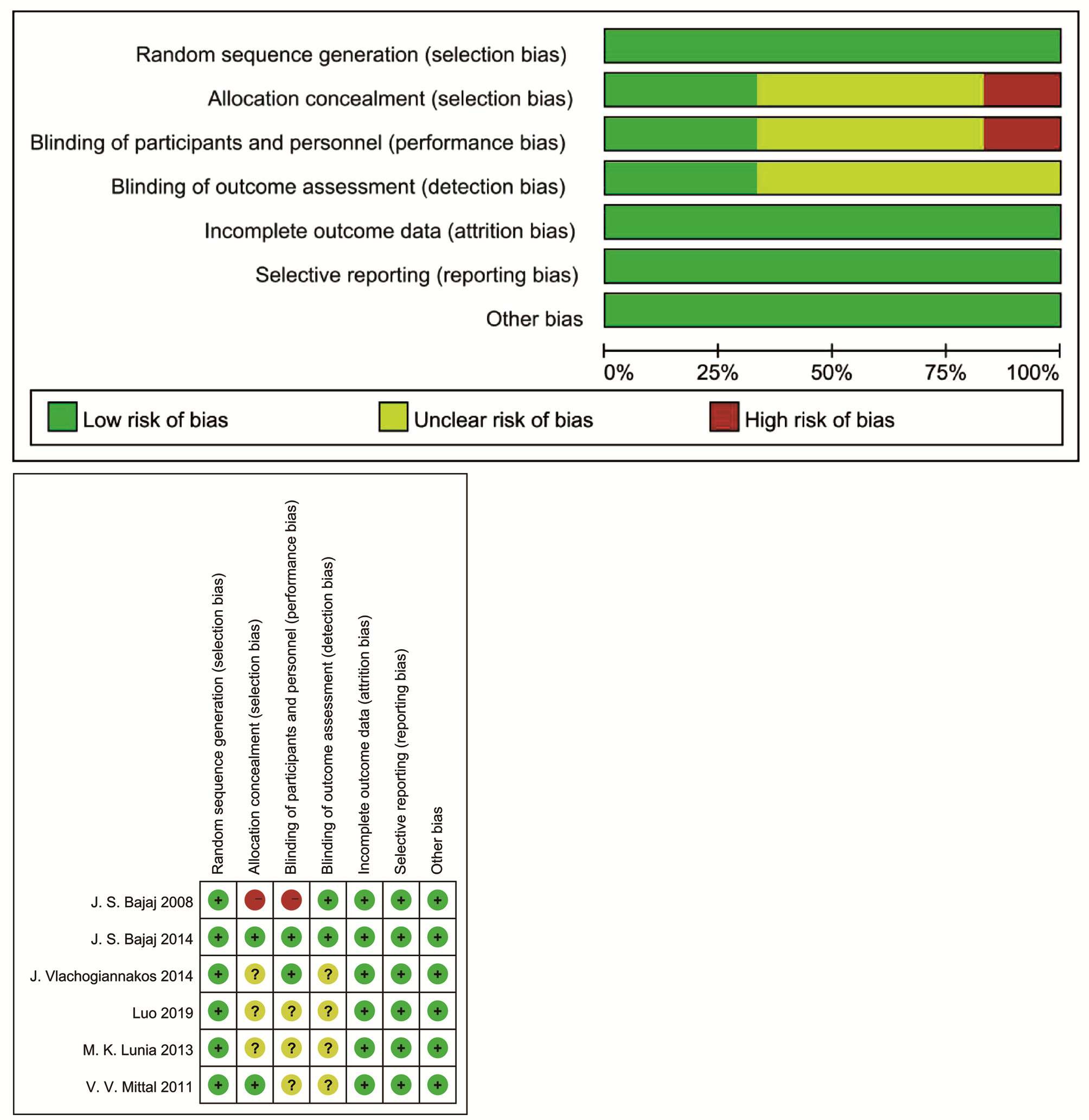
对照组:无治疗8周 ①②③④⑥⑦⑧⑨ 纳入研究及时间 地区 研究类型 研究人数(E∶C) MHE诊断指标 罗兰[19](2019) 中国 RCT 109(56∶53) NCT、DST Bajaj等[14](2014) 美国 RCT 37(18∶19) NCT、DST、BDT Vlachogiannakos等[18](2014) 希腊 RCT 72(37∶35) NCT、BEAP Lunia等[15](2013) 印度 RCT 81(42∶39) PHES≤5 Mittal等[17](2011) 印度 RCT 80(40∶40) NCT、FCT Bajaj等[13](2008) 美国 RCT 25(17∶8) NCT、BDT、DST 注:E,干预组;C,对照组;NCT,数字连接测试;DST,数字符号试验;BDT,block design test;PHES,HE智力测试评分;BEAP,brainstem auditory evoked potentials;FCT,figure connection test。结局指标包括①OHE发生率;②MHE逆转率;③神经心理学测试;④血氨;⑤ALT、AST;⑥炎性指标;⑦生活质量;⑧Child-Pugh评分;⑨MELD评分;⑩肠道菌群;B11不良事件发生。 -
[1] VILSTRUP H, AMODIO P, BAJAJ J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver[J]. Hepatology, 2014, 60(2): 715-735. DOI: 10.1002/hep.27210. [2] DHIMAN RK, SARASWAT VA, SHARMA BK, et al. Minimal hepatic encephalopathy: consensus statement of a working party of the Indian National Association for Study of the Liver[J]. J Gastroenterol Hepatol, 2010, 25(6): 1029-1041. DOI: 10.1111/j.1440-1746.2010.06318.x. [3] Chinese Society of Hepatology, Chinese Medical Association. Guidelines on the management of hepatic encephalopathy in cirrhosis[J]. J Clin Hepatol, 2018, 34(10): 2076-2089. DOI: 10.3969/j.issn.1001-5256.2018.10.007.中华医学会肝病学分会. 肝硬化肝性脑病诊疗指南[J]. 临床肝胆病杂志, 2018, 34(10): 2076-2089. DOI: 10.3969/j.issn.1001-5256.2018.10.007. [4] AMODIO P, DEL PICCOLO F, MARCHETTI P, et al. Clinical features and survivial of cirrhotic patients with subclinical cognitive alterations detected by the number connection test and computerized psychometric tests[J]. Hepatology, 1999, 29(6): 1662-1667. DOI: 10.1002/hep.510290619. [5] HARTMANN IJ, GROENEWEG M, QUERO JC, et al. The prognostic significance of subclinical hepatic encephalopathy[J]. Am J Gastroenterol, 2000, 95(8): 2029-2034. DOI: 10.1111/j.1572-0241.2000.02265.x. [6] INOUE E, HORI S, NARUMI Y, et al. Portal-systemic encephalopathy: presence of basal ganglia lesions with high signal intensity on MR images[J]. Radiology, 1991, 179(2): 551-555. DOI: 10.1148/radiology.179.2.2014310. [7] ROMERO-GÓMEZ M, BOZA F, GARCÍA-VALDECASAS MS, et al. Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy[J]. Am J Gastroenterol, 2001, 96(9): 2718-2723. DOI: 10.1111/j.1572-0241.2001.04130.x. [8] ROMERO-GÓMEZ M, CÓRDOBA J, JOVER R, et al. Value of the critical flicker frequency in patients with minimal hepatic encephalopathy[J]. Hepatology, 2007, 45(4): 879-885. DOI: 10.1002/hep.21586. [9] SAXENA N, BHATIA M, JOSHI YK, et al. Electrophysiological and neuropsychological tests for the diagnosis of subclinical hepatic encephalopathy and prediction of overt encephalopathy[J]. Liver, 2002, 22(3): 190-197. DOI: 10.1034/j.1600-0676.2002.01431.x. [10] DHIMAN RK, KURMI R, THUMBURU KK, et al. Diagnosis and prognostic significance of minimal hepatic encephalopathy in patients with cirrhosis of liver[J]. Dig Dis Sci, 2010, 55(8): 2381-2390. DOI: 10.1007/s10620-010-1249-7. [11] FICHET J, MERCIER E, GENÉE O, et al. Prognosis and 1-year mortality of intensive care unit patients with severe hepatic encephalopathy[J]. J Crit Care, 2009, 24(3): 364-370. DOI: 10.1016/j.jcrc.2009.01.008. [12] SCHIFF ER, MADDREY WC, REDDY KR. Schiff's diseases of the liver[M]. REN H, translate. Beijing: China Science and Technology Press, 2021: 6.SCHIFF ER, MADDREY WC, REDDY KR. SCHIFF. 肝脏病学[M]. 任红, 主译. 北京: 中国科学技术出版社, 2021: 6. [13] BAJAJ JS, SAEIAN K, CHRISTENSEN KM, et al. Probiotic yogurt for the treatment of minimal hepatic encephalopathy[J]. Am J Gastroenterol, 2008, 103(7): 1707-1715. DOI: 10.1111/j.1572-0241.2008.01861.x. [14] BAJAJ JS, HEUMAN DM, HYLEMON PB, et al. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis[J]. Aliment Pharmacol Ther, 2014, 39(10): 1113-1125. DOI: 10.1111/apt.12695. [15] LUNIA MK. A randomised controlled trial on efficacy of probiotics on reversal of minimal hepatic encephalopathy and its correlation with improvement in small intestinal bacterial overgrowth and orocecal transit time in patients with cirrhosis[J]. United Eur Gastroent, 2013, 1(1): A153-A154. DOI: 10.1177/2050640613502900. [16] LUNIA MK, SHARMA BC, SHARMA P, et al. Probiotics prevent hepatic encephalopathy in patients with cirrhosis: a randomized controlled trial[J]. Clin Gastroenterol Hepatol, 2014, 12(6): 1003-1008. e1. DOI: 10.1016/j.cgh.2013.11.006. [17] MITTAL VV, SHARMA BC, SHARMA P, et al. A randomized controlled trial comparing lactulose, probiotics, and L-ornithine L-aspartate in treatment of minimal hepatic encephalopathy[J]. Eur J Gastroenterol Hepatol, 2011, 23(8): 725-732. DOI: 10.1097/MEG.0b013e32834696f5. [18] VLACHOGIANNAKOS J, VASIANOPOULOU P, VIAZIS N, et al. The role of probiotics in the treatment of minimal hepatic encephalopathy. A prospective, randomized placebo-controlled, double-blind study[J]. Hepatology, 2014, 60: 376A. DOI: 10.1002/hep.27498. [19] LUO L. A study on the effect of probiotics on patients with mild hepatic encephalopathy in liver cirrhosis[D]. Luzhou: Southwest Medical University, 2019.罗兰. 益生菌对肝硬化轻微肝性脑病患者的作用的研究[D]. 泸州: 西南医科大学, 2019. [20] SHARMA K, PANT S, MISRA S, et al. Effect of rifaximin, probiotics, and l-ornithine l-aspartate on minimal hepatic encephalopathy: a randomized controlled trial[J]. Saudi J Gastroenterol, 2014, 20(4): 225-232. DOI: 10.4103/1319-3767.136975. [21] XIA X, CHEN J, XIA J, et al. Role of probiotics in the treatment of minimal hepatic encephalopathy in patients with HBV-induced liver cirrhosis[J]. J Int Med Res, 2018, 46(9): 3596-3604. DOI: 10.1177/0300060518776064. [22] PRATAP MOULI V, BENJAMIN J, BHUSHAN SINGH M, et al. Effect of probiotic VSL#3 in the treatment of minimal hepatic encephalopathy: A non-inferiority randomized controlled trial[J]. Hepatol Res, 2015, 45(8): 880-889. DOI: 10.1111/hepr.12429. [23] SHAVAKHI A, HASHEMI H, TABESH E, et al. Multistrain probiotic and lactulose in the treatment of minimal hepatic encephalopathy[J]. J Res Med Sci, 2014, 19(8): 703-708. [24] YU FJ. Effect of lactulose combined with triplet viable bifidobacteria capsules on mild hepatic encephalopathy patients[J]. Chin J Microecol, 2020, 32(8): 929-932. DOI: 10.13381/j.cnki.cjm.202008014.余芳杰. 乳果糖联合双歧杆菌三联活菌胶囊在轻微性肝性脑病患者中的应用效果[J]. 中国微生态学杂志, 2020, 32(8): 929-932. DOI: 10.13381/j.cnki.cjm.202008014. [25] LIU RM, LI L, XIE JT. Protective effect of bifidobacterium quadruple viable tablet combined with lactulose on inflammatory injury of intestinal mucosa in patients with mild hepatic encephalopathy[J]. Chin J Microecol, 2021, 33(10): 1181-1184. DOI: 10.13381/j.cnki.cjm.202110012.刘荣明, 李亮, 解君挺. 双歧杆菌四联活菌片联合乳果糖对轻微型肝性脑病患者炎症性肠黏膜损伤的保护作用[J]. 中国微生态学杂志, 2021, 33(10): 1181-1184. DOI: 10.13381/j.cnki.cjm.202110012. [26] MUKHERJEE S, JOHN S. Lactulose[M]//StatPearls. Treasure Island (FL): StatPearls Publishing, 2021. -



 PDF下载 ( 3743 KB)
PDF下载 ( 3743 KB)

 下载:
下载: