基于超声造影LI-RADS特征的肝细胞癌微血管侵犯列线图模型的构建及验证
DOI: 10.3969/j.issn.1001-5256.2022.11.016
Construction and validation of a nomogram model for microvascular invasion in hepatocellular carcinoma based on the characteristics on contrast-enhanced ultrasound Liver Imaging Reporting and Data System
-
摘要:
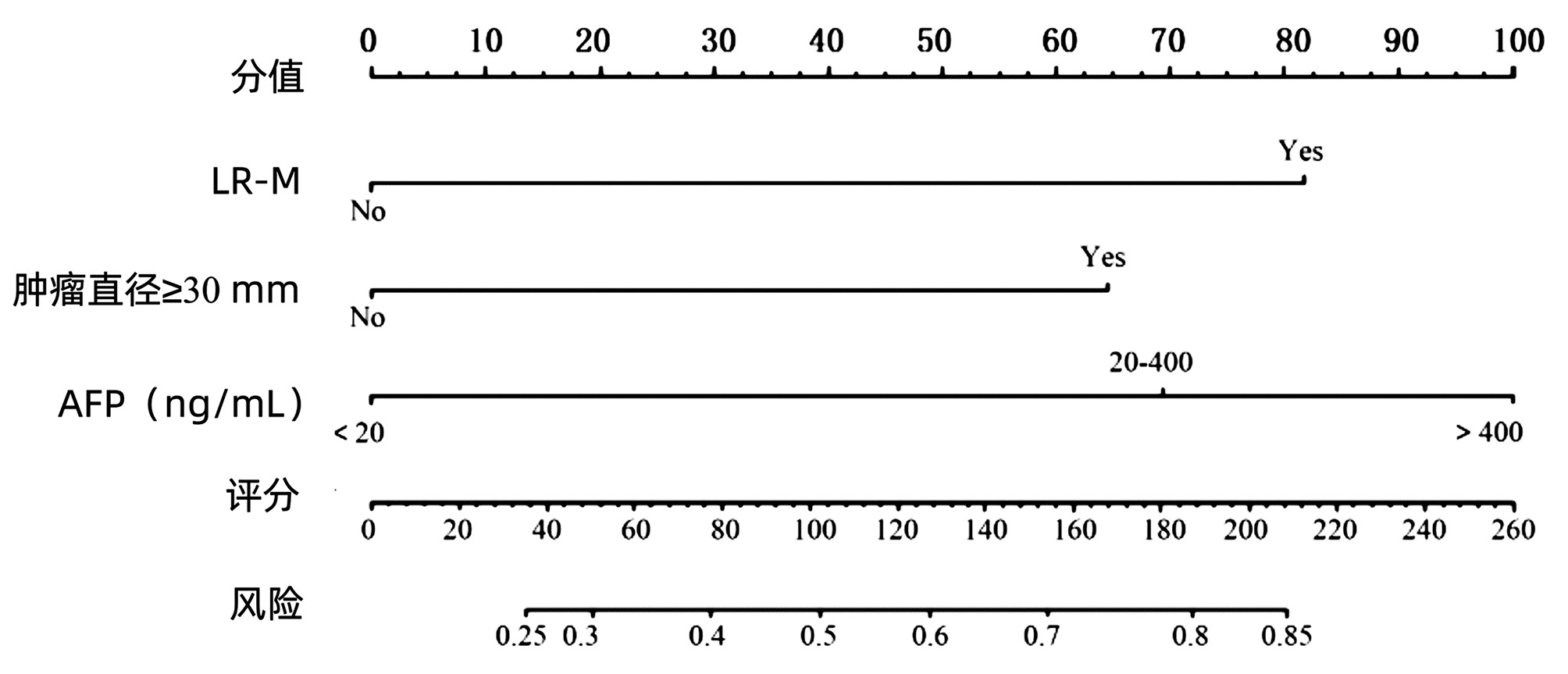
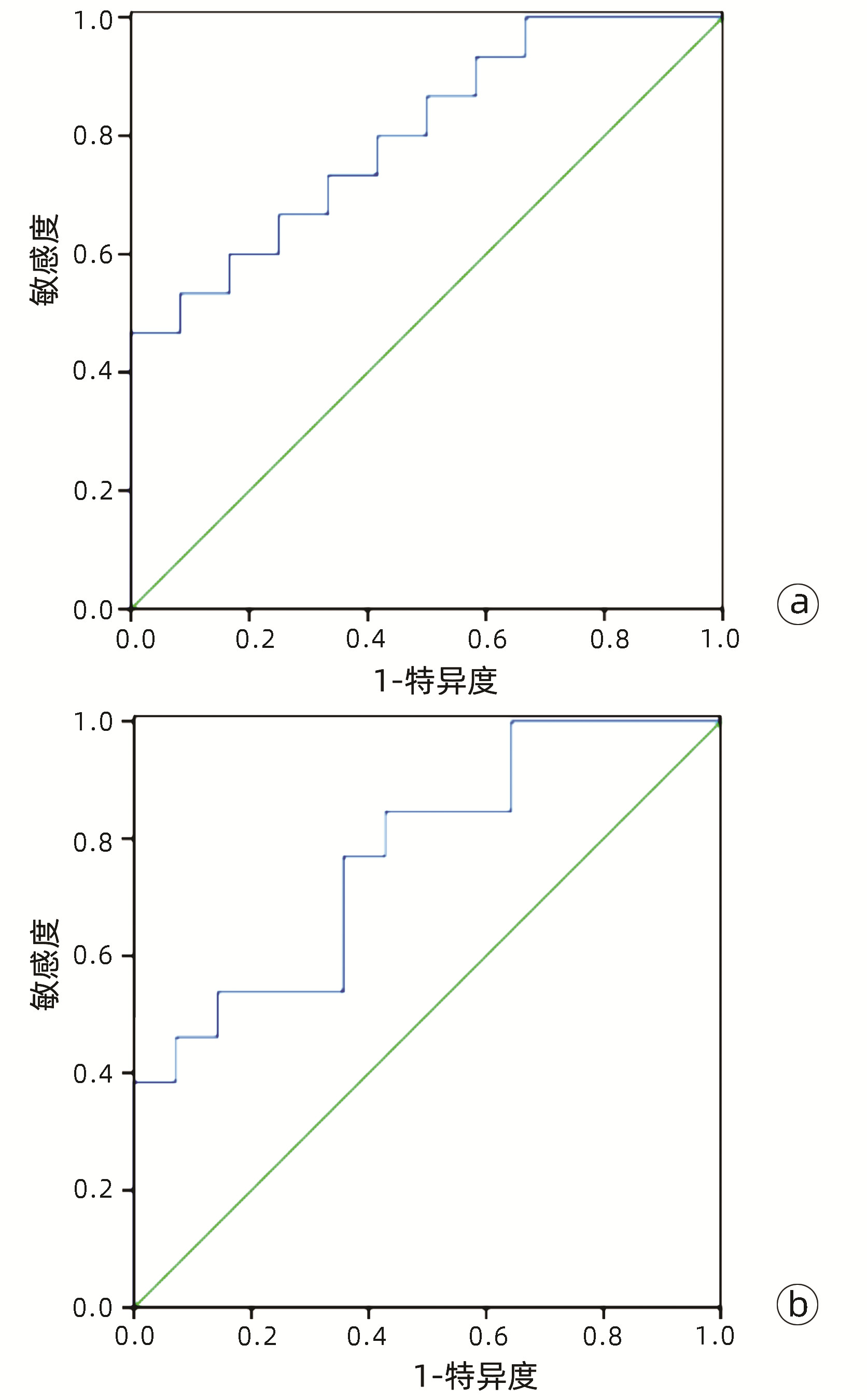
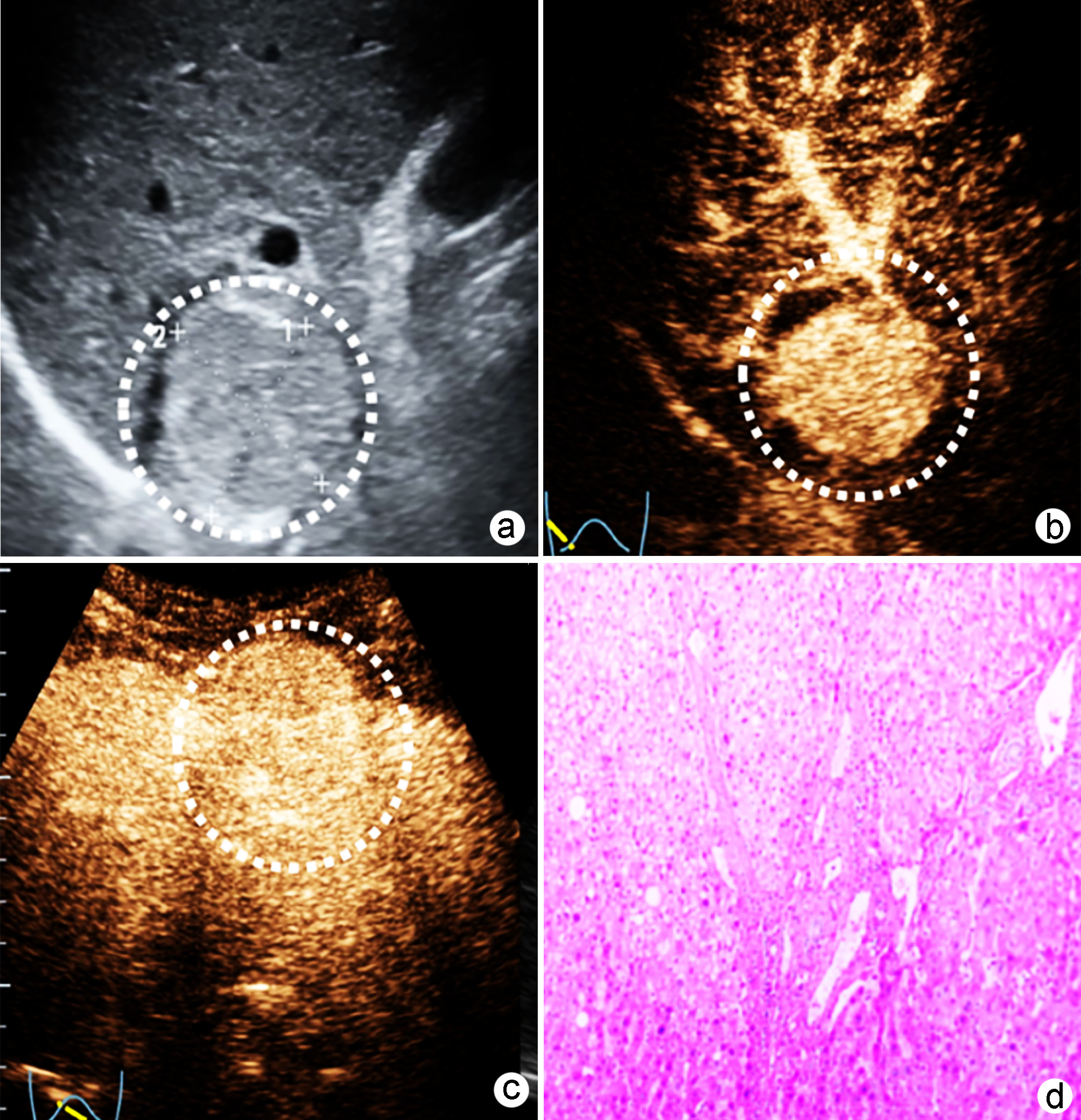
目的 基于肝细胞癌(HCC)患者的超声造影(CEUS)肝脏成像报告和数据系统(LI-RADS)特征建立预测微血管侵犯(MVI)的列线图模型并进行验证。 方法 选取2017年1月—2020年7月在江苏大学附属武进医院确诊的HCC患者共262例,按照1∶ 1比例随机分为建模组和验证组各131例,以术后镜下病理结果确诊MVI,其中建模组MVI 70例和验证组MVI 56例。采用超声造影评估两组的LI-RADS特征。两组间计量资料比较采用独立样本t检验;两组间计数资料比较采用χ2检验。采用单因素和多因素Logistic回归分析筛选建模组MVI的危险因素;绘制受试者工作特征曲线(ROC曲线),计算模型预测MVI的曲线下面积(AUC),评估预测准确度;应用决策曲线分析模型的一致性,比较模型预测MVI的校正曲线与标准曲线的离散度。 结果 建模组与验证组患者的临床资料和CEUS检查结果比较,差异均无统计学意义(P值均>0.05)。单因素分析显示,与MVI阴性患者相比,MVI阳性患者血清AFP水平显升高,肿瘤直径增大,LI-RADS显示LR-5“后出”和LR-M“先出”增多,LI-RADS分级较高,差异均有统计学意义(P值均<0.05)。多因素分析显示,AFP 20~400 ng/mL(OR=2.65,P<0.001)、AFP≥400 ng/mL(OR=3.98,P<0.001)、肿瘤直径≥30 mm(OR=2.12,P<0.001)和CEUS显示LR-M(OR=3.24,P<0.001) 是MVI的独立危险因素。ROC曲线显示,列线图预测建模组和验证组MVI的AUC分别为0.867和0.821。列线图模型的一致性指数C-Index为0.765(95%CI:0.701~0.834)。在建模组和验证组,列线图模型的预测校准曲线与标准曲线均接近。 结论 利用CEUS得出LI-RADS,并结合AFP和肿瘤直径建立的列线图模型有较好的应用价值,有助于指导临床术前筛选MVI高危患者,制订恰当的手术方案。 Abstract:Objective To construct and validate a nomogram model for predicting microvascular invasion (MVI) based on the characteristics on contrast-enhanced ultrasound (CEUS) Liver Imaging Reporting and Data System (LI-RADS) in patients with hepatocellular carcinoma (HCC). Methods A total of 262 patients with HCC who were diagnosed in Wujin Hospital Affiliated to Jiangsu University from January 2017 to July 2020 were enrolled and randomly divided into modeling group and validation group at a ratio of 1∶ 1, with 131 patients in each group. MVI was confirmed by postoperative microscopic pathological results, and there were 70 patients with MVI in the modeling group and 56 patients with MVI in the validation group. CEUS was used to evaluate LI-RADS characteristics for the two groups. The independent samples t-test was used for comparison of continuous data between the two groups, and the chi-square test was used for comparison of categorical data between the two groups. Univariate and multivariate Logistic regression analyses were used to identify the risk factors for MVI in the modeling group; the receiver operating characteristic (ROC) curve was plotted, and the area under the ROC curve (AUC) was calculated for the model in predicting MVI to evaluate the accuracy of prediction; a decision curve analysis was used to evaluate the consistency of the model, and dispersion was compared between the calibration curve and the standard curve for the model in predicting MVI. Results There were no significant differences in clinical data and CEUS findings between the modeling group and the validation group (all P > 0.05). The univariate analysis showed that compared with the MVI-negative patients, the MVI-positive patients had significant increases in serum alpha fetoprotein (AFP) level, tumor diameter, and LR-5 "late and mild washout" and LR-M "early washout" on LI-RADS, as well as a significantly higher LI-RADS grade (all P < 0.05). The multivariate analysis showed that AFP 20-400 ng/mL (odds ratio [OR]=2.65, P < 0.001), AFP≥400 ng/mL (OR=3.98, P < 0.001), tumor diameter ≥30 mm (OR=2.12, P < 0.001), and LR-M on CEUS (OR=3.24, P < 0.001) were independent risk factors for MVI. The ROC curve analysis showed that the nomogram had an AUC of 0.867 and 0.821 in predicting MVI in the modeling group and the validation group, respectively. The nomogram model had a C-Index of 0.765 (95% confidence interval: 0.701-0.834). The calibration curves of the nomogram model were close to the standard curve in both groups. Conclusion The nomogram model based on LI-RADS obtained by CEUS in combination with AFP and tumor diameter has a good application value and can guide the preoperative screening for patients at a high risk of MVI and the development of appropriate surgical plans in clinical practice. -
表 1 建模组与验证组的临床资料和CEUS特征比较
Table 1. Comparison of clinical datas and CEUS characteristics between model group and validation group
指标 建模组(n=131) 验证组(n=131) 统计值 P值 男/女(例) 78/53 70/61 χ2=0.994 0.319 年龄(岁) 58.2±6.7 57.2±6.3 t=1.052 0.198 乙型肝炎[例(%)] 115(87.8) 110(84.0) χ2=0.787 0.375 肝硬化[例(%)] 80(61.1) 84(64.1) χ2=0.261 0.610 肿瘤TNM分期[例(%)] χ2=0.138 0.710 Ⅰ~Ⅱ 60(45.8) 63(48.1) Ⅲ~Ⅳ 71(54.2) 68(51.9) 分化级别[例(%)] χ2=0.245 0.621 低 65(49.6) 61(46.6) 中高 66(50.4) 70(53.4) MVI[例(%)] 70(53.4) 56(42.7) χ2=2.997 0.083 血生化指标 PT>14 s[例(%)] 42(32.1) 33(25.2) χ2=1.513 0.219 AFP(ng/mL) 365±74 349±68 t=0.795 0.302 PLT(×109/L) 201±32 212±39 t=0.632 0.348 TBil(μmol/L) 15.6±3.8 13.9±3.2 t=0.469 0.521 Alb(g/L) 35.9±4.5 33.2±4.3 t=0.396 0.627 ALT(U/L) 40.2±5.3 43.9±5.7 t=0.559 0.501 二维超声 肿瘤直径≥30 mm[例(%)] 92(70.2) 81(61.8) χ2=2.059 0.151 回声类型[例(%)] χ2=0.177 0.674 低 95(72.5) 98(74.8) 等或高 36(27.5) 33(25.2) 边缘不清[例(%)] 101(77.1) 93(71.0) χ2=1.271 0.260 不规则外形[例(%)] 92(70.2) 94(71.8) χ2=0.074 0.785 Halo征[例(%)] 32(24.4) 28(21.4) χ2=0.346 0.556 血管分布[例(%)] χ2=0.078 0.780 少 97(74.0) 95(72.5) 多 34(26.0) 36(27.5) CEUS特征[例(%)] LR-5动脉期增强 93(71.0) 89(67.9) χ2=0.288 0.592 “后出”征象 57(43.5) 51(38.9) χ2=0.567 0.451 LR-M边缘增强 19(14.5) 22(16.8) χ2=0.260 0.610 “先出”征象 78(59.5) 71(54.2) χ2=0.762 0.383 LI-RADS分级[例(%)] χ2=0.492 0.782 3~4 16(12.3) 19(14.5) 5 48(36.6) 50(38.2) M 67(51.1) 62(47.3) 镶嵌图形征[例(%)] 23(17.6) 25(19.1) χ2=0.102 0.749 结节内结节征[例(%)] 20(15.3) 17(13.0) χ2=0.283 0.595 表 2 MVI的危险因素分析
Table 2. Risk factor analysis of MVI
因素 单因素分析 多因素分析 OR 95%CI P值 OR 95%CI P值 AFP 20~400 ng/mL 3.19 2.66~3.42 <0.001 2.65 2.13~3.23 <0.001 ≥400 ng/mL 4.45 3.78~4.79 <0.001 3.98 3.32~4.39 <0.001 肿瘤直径≥30 mm 2.76 2.23~3.16 <0.001 2.12 1.69~2.58 <0.001 LR-5“后出” 2.09 1.56~2.54 <0.001 LR-M“先出” 3.96 3.46~4.49 <0.001 3.24 2.78~3.65 <0.001 LI-RADS分级 2.01 1.48~2.43 <0.001 -
[1] HE YZ, HE K, HUANG RQ, et al. Preoperative evaluation and prediction of clinical scores for hepatocellular carcinoma microvascular invasion: a single-center retrospective analysis[J]. Ann Hepatol, 2020, 19(6): 654-661. DOI: 10.1016/j.aohep.2020.07.002. [2] LIU ZY, WU D, OU JL, et al. Characteristics and related clinical indicators of microvascular invasion in hepatocellular carcinoma[J] J Hepatopancreatobiliary Surg, 2017, 29(2): 107-111. DOI: 10.11952/j.issn.1007-1954.2017.02.005.刘臻玉, 武丹, 区锦玲, 等. 肝癌微血管侵犯的特点及相关临床指标[J]. 肝胆胰外科杂志, 2017, 29(2): 107-111. DOI: 10.11952/j.issn.1007-1954.2017.02.005. [3] ERSTAD DJ, TANABE KK. Prognostic and therapeutic implications of microvascular invasion in hepatocellular carcinoma[J]. Ann Surg Oncol, 2019, 26(5): 1474-1493. DOI: 10.1245/s10434-019-07227-9. [4] ZENG YM, LIU D, TANG CL, et al. Clinical value of contrast-enhanced ultrasound and gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging in the diagnosis of hepatocellular carcinoma[J]. Chin J Dig Surg, 2020, 19(10): 1098-1107. DOI: 10.3760/cma.j.cn115610-20200921-00628.曾杨媚, 刘灯, 唐春霖, 等. 超声造影与钆塞酸二钠增强磁共振检查诊断肝细胞癌的临床价值[J]. 中华消化外科杂志, 2020, 19(10): 1098-1107. DOI: 10.3760/cma.j.cn115610-20200921-00628. [5] LI R, FAN HJ, XU J, et al. The predictive value of contrast-enhanced ultrasound, serum AFP and CEA levels on microvascular invasion and early recurrence of liver cancer after interventional surgery[J]. Pract J Cancer, 2021, 36 (3): 452-456. DOI: 10.3969/j.issn.1001-5930.2021.03.027.李冉, 范会军, 徐杰, 等. 超声造影、血清AFP、CEA水平对肝癌介入术后微血管侵犯、早期复发的预测价值[J]. 实用癌症杂志, 2021, 36(3): 452-456. DOI: 10.3969/j.issn.1001-5930.2021.03.027. [6] DIETRICH CF, NOLSØE CP, BARR RG, et al. Guidelines and good clinical practice recommendations for Contrast-Enhanced Ultrasound (CEUS) in the liver-update 2020 WFUMB in cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS[J]. Ultrasound Med Biol, 2020, 46(10): 2579-2604. DOI: 10.1016/j.ultrasmedbio.2020.04.030. [7] WU QY, LIU J, YANG CS, et al. Application value of imaging examinations in the diagnosis of small hepatocellular carcinoma[J]. Chin J Dig Surg, 2022, 21(4): 543-550. DOI: 10.3760/cma.j.cn115610-20220321-00146.伍秋艳, 刘娟, 杨崇双, 等. 影像学检查在诊断小肝癌中的应用价值[J]. 中华消化外科杂志, 2022, 21(4): 543-550. DOI: 10.3760/cma.j.cn115610-20220321-00146. [8] WU JY, BAI XM, WANG H, et al. The perfusion features of recurrent hepatocellular carcinoma after radiofrequency ablation using contrast-enhanced ultrasound and pathological stemness evaluation: compared to initial tumors[J]. Front Oncol, 2020, 10: 1464. DOI: 10.3389/fonc.2020.01464. [9] DONG Y, QIU Y, YANG D, et al. Potential application of dynamic contrast enhanced ultrasound in predicting microvascular invasion of hepatocellular carcinoma[J]. Clin Hemorheol Microcirc, 2021, 77(4): 461-469. DOI: 10.3233/CH-201085. [10] FAN PL, DING H, MAO F, et al. Enhancement patterns of small hepatocellular carcinoma (≤ 30 mm) on contrast-enhanced ultrasound: Correlation with clinicopathologic characteristics[J]. Eur J Radiol, 2020, 132: 109341. DOI: 10.1016/j.ejrad.2020.109341. [11] KIM YY, KIM MJ, KIM EH, et al. Hepatocellular carcinoma versus other hepatic malignancy in cirrhosis: Performance of LI-RADS version 2018[J]. Radiology, 2019, 291(1): 72-80. DOI: 10.1148/radiol.2019181995. [12] LEI Z, LI J, WU D, et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma within the Milan criteria[J]. JAMA Surg, 2016, 151(4): 356-363. DOI: 10.1001/jamasurg.2015.4257. [13] GRANATA V, FUSCO R, SETOLA SV, et al. Microvascular invasion and grading in hepatocellular carcinoma: correlation with major and ancillary features according to LIRADS[J]. Abdom Radiol (NY), 2019, 44(8): 2788-2800. DOI: 10.1007/s00261-019-02056-6. [14] SUN Z, SHAO WW, SONG JH. Progress in diagnosis and treatment of hepatocellular carcinoma with microvascular invasion[J/CD]. Chin J Hepat Surg(Electronic Edition), 2021, 10(3): 235-241.孙振, 邵巍伟, 宋京海. 肝细胞癌合并微血管侵犯的诊疗进展[J/CD]. 中华肝脏外科手术学电子杂志, 2021, 10(3): 235-241. [15] LIU LF, LIU JJ, LUO T, et al. Value of preoperative ultrasound combined with serum alpha fetoprotein in predicting microvascular invasion of hepatocellular carcinoma[J]. J Guangxi Med Univ, 2018, 35(9): 1260-1263. DOI: 10.16190/j.cnki.45-1211/r.2018.09.019.刘连凤, 刘军杰, 罗涛, 等. 术前超声联合血清甲胎蛋白水平预测肝癌微血管侵犯的价值[J]. 广西医科大学学报, 2018, 35(9): 1260-1263. DOI: 10.16190/j.cnki.45-1211/r.2018.09.019. [16] JIN Y, LI JT. Research progress on clinical related factors and molecular markers of microvascular invasion by liver cancer cells[J]. J Clin Hepatol, 2013, 29(7): 550-553. DOI: 10.3969/j.issn.1001-5256.2013.07.020.金赟, 李江涛. 肝癌细胞侵犯微血管的临床相关因素及分子标志物的研究进展[J]. 临床肝胆病杂志, 2013, 29(7): 550-553. DOI: 10.3969/j.issn.1001-5256.2013.07.020. [17] KIM SS, LEE S, KIM MJ. Prognostic factors of gadoxetic acid-enhanced MRI for postsurgical outcomes in multicentric hepatocellular carcinoma[J]. Eur Radiol, 2021, 31(5): 3405-3416. DOI: 10.1007/s00330-020-07419-y. [18] KONO Y, LYSHCHIK A, COSGROVE D, et al. Contrast Enhanced Ultrasound (CEUS) Liver Imaging Reporting and Data System (LI-RADS®): the official version by the American College of Radiology (ACR)[J]. Ultraschall Med, 2017, 38(1): 85-86. DOI: 10.1055/s-0042-124369. [19] ZHOU H, ZHANG C, DU L, et al. Contrast-enhanced ultrasound liver imaging reporting and data system in diagnosing hepatocellular carcinoma: Diagnostic performance and interobserver agreement[J]. Ultraschall Med, 2022, 43(1): 64-71. DOI: 10.1055/a-1168-6321. [20] ZHU W, QING X, YAN F, et al. Can the contrast-enhanced ultrasound washout rate be used to predict microvascular invasion in hepatocellular carcinoma?[J]. Ultrasound Med Biol, 2017, 43(8): 1571-1580. DOI: 10.1016/j.ultrasmedbio.2017.04.003. [21] CARR BI, INCE V, BAG HG, et al. Microscopic vascular invasion by hepatocellular carcinoma in liver transplant patients[J]. Clin Pract (Lond), 2020, 17(3): 1497-1505. [22] WANG P, NIE F, DONG T, et al. Study on correlation between two-dimensional ultrasound, contrast-enhanced ultrasound and microvascular invasion in hepatocellular carcinoma[J]. Clin Hemorheol Microcirc, 2022, 80(2): 97-106. DOI: 10.3233/CH-211190. -



 PDF下载 ( 2938 KB)
PDF下载 ( 2938 KB)

 下载:
下载: