慢性乙型肝炎患者合并极轻肝脏脂肪变性的临床特征及危险因素分析
DOI: 10.3969/j.issn.1001-5256.2023.01.010
Clinical features and related risk factors of chronic hepatitis B patients with concomitant minimal hepatic steatosis
-
摘要:
目的 观察慢性乙型肝炎(CHB)患者合并极轻肝脏脂肪变性的临床指标的变化,并分析极轻脂肪变性发生的相关影响因素。 方法 纳入2018年7月—2022年3月在南京大学医学院附属鼓楼医院感染疾病科行肝穿刺活检的CHB患者共179例。依据脂肪变性程度分为无脂肪变性组(n=98)、极轻脂肪变性组(n=81)。收集人口学信息、临床资料及肝组织病理学资料,比较各观察指标在两组中的差异。符合正态分布的计量资料两组间比较采用独立样本t检验,不符合正态分布的两组间比较采用Mann-Whitney U检验,计数资料两组间比较采用χ2检验。相关性分析采用Spearman检验。Logistic回归分析极轻脂肪变性发生的危险因素。 结果 极轻脂肪变性组中男性比例(69.1% vs 52.0%)及显著纤维化比例(43.2% vs 25.5%)较无脂肪变性组高(χ2值分别为5.390、6.234,P值均<0.05)。极轻脂肪变性组BMI[(23.61±2.95)kg/m2 vs(22.13±2.67)kg/m2]、尿酸(UA)[333.0(291.0~375.5)μmol/L vs 287.5(244.8~345.3)μmol/L]、TG[0.92(0.66~1.14)μmol/L vs 0.77(0.62~1.02)μmol/L]、肝脏脂肪受控衰减参数(CAP)[234(214~258)dB/m vs 218(201~237)dB/m]水平均高于无脂肪变性组(t=-4.150,Z=-3.620,Z=-2.224,Z=-2.867,P值均<0.05)。正常体质量组,极轻脂肪变性患者UA[(333.0±63.9)μmol/L vs(291.0±72.8)μmol/L]、HBV DNA[4.44(3.51~6.79)log10 IU/mL vs 3.42(3.00~5.03)log10 IU/mL]高于无脂肪变性患者(t=-2.395,Z=-2.474,P值均<0.05)。BMI(OR=1.223,95%CI:1.086~1.378,P=0.001)、UA(OR=1.006,95%CI:1.002~1.010,P=0.008)为CHB合并极轻脂肪变性的危险因素。UA(OR=1.007,95%CI:1.001~1.013,P=0.022)为正常体质量CHB患者合并极轻脂肪变性的危险因素。 结论 合并极轻脂肪变性的CHB患者显著纤维化比例、CAP高于无脂肪变性者。BMI、UA是CHB患者发生极轻脂肪变性的独立危险因素。尤其在正常体质量CHB患者,UA升高与极轻脂肪变性的发生关系密切。 Abstract:Objective To investigate the changes of clinical indices in chronic hepatitis B (CHB) patients with concomitant minimal hepatic steatosis and related factors for minimal hepatic steatosis. Methods A total of 179 CHB patients who underwent liver biopsy in Department of Infectious Diseases, Affiliated Drum Tower Hospital of Nanjing University Medical School, from July 2018 to March 2022 were enrolled, and according to the degree of steatosis, they were divided into non-steatosis group with 98 patients and minimal hepatic steatosis group with 81 patients. Demographic information, clinical data, and liver histopathology data were collected, and related observation indices were compared between the two groups. The independent samples t-test was used for comparison of normally distributed continuous data between two groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between two groups; the chi-square test was used for comparison of categorical data between groups. A Spearman correlation analysis was performed, and a Logistic regression analysis was used to investigate the risk factors for minimal hepatic steatosis. Results Compared with the non-steatosis group, the minimal hepatic steatosis group had a significantly higher proportion of male patients (69.1% vs 52.0%, χ2=5.390, P < 0.05) and a significantly higher proportion of patients with significant liver fibrosis (43.2% vs 25.5%, χ2=6.234, P < 0.05). Compared with the non-steatosis group, the minimal hepatic steatosis group had significantly higher levels of body mass index (BMI) (23.61±2.95 kg/m2 vs 22.13±2.67 kg/m2, t=-4.150, P < 0.05), uric acid (UA) [333.0(291.0-375.5) μmol/L vs 287.5(244.8-345.3) μmol/L, Z=-3.620, P < 0.05], triglyceride [0.92 (0.66-1.14) μmol/L vs 0.77 (0.62-1.02) μmol/L, Z=-2.224, P < 0.05], and controlled attenuation parameter (CAP) [234 (214-258) dB/m vs 218 (201-237) dB/m, Z=-2.867, P < 0.05]. In the group with normal body weight, the patients with minimal hepatic steatosis had significantly higher levels of UA (333.0±63.9 μmol/L vs 291.0±72.8 μmol/L, t=-2.395, P < 0.05) a nd HBV DNA [4.44 (3.51-6.79) log10 IU/mL vs 3.42 (3.00-5.03) log10 IU/mL, Z=-2.474, P < 0.05]. BMI (odds ratio [OR]=1.223, 95% confidence interval [CI] : 1.086-1.378, P=0.001) and UA (OR=1.006, 95%CI: 1.002-1.010, P=0.008) were risk factors for minimal hepatic steatosis in CHB patients, and UA (OR=1.007, 95%CI: 1.001-1.013, P=0.022) was a risk factors for minimal hepatic steatosis in CHB patients with normal body weight. Conclusion Compared with the non-steatosis CHB patients, the CHB patients with minimal hepatic steatosis have a significantly higher proportion of patients with significant liver fibrosis and a significantly higher level of CAP. BMI and UA are independent risk factors for minimal hepatic steatosis in CHB patients, and for the CHB patients with normal body weight, elevated UA is closely associated with the onset of minimal hepatic steatosis. -
Key words:
- Hepatitis B, Chronic /
- Non-alcoholic Fatty Liver Disease /
- Risk Factor
-
表 1 CHB患者的临床及病理特征
Table 1. Baseline clinical characteristics of the CHB patients
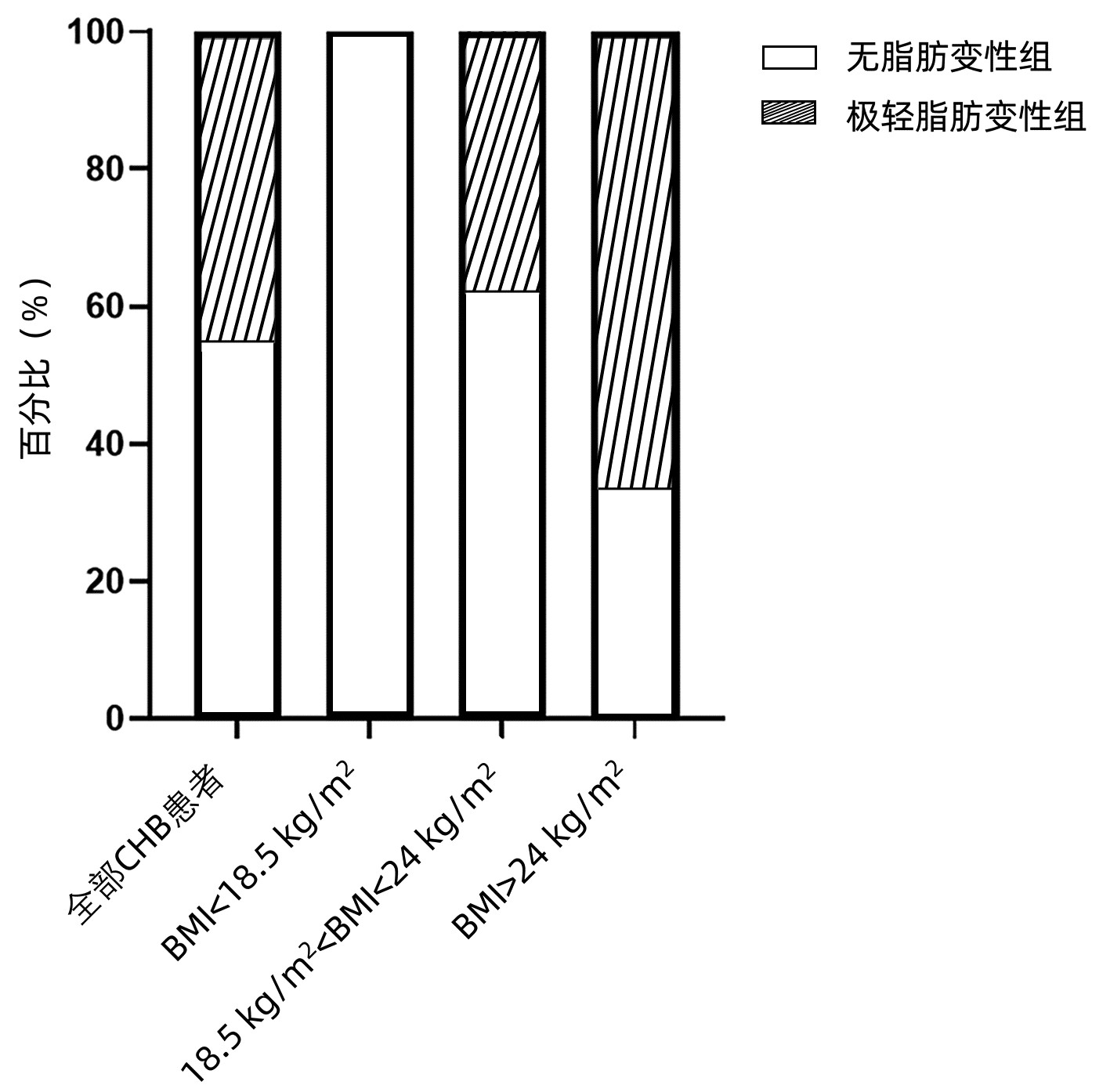
指标 无脂肪变性组(n=98) 极轻脂肪变性组(n=81) 统计值 P值 男[例(%)] 51(52.0) 56(69.1) χ2=5.390 0.020 年龄(岁) 38.0(31.0~48.3) 37.0(31.5~47.0) Z=-0.072 0.942 高血压[例(%)] 2(2.0) 5(6.2) χ2=2.004 0.157 BMI(kg/m2) 22.13±2.67 23.61±2.95 t=-4.150 <0.001 PLT(×109/L) 169.0±47.8 165.5±48.6 t=0.732 0.465 ALT(U/L) 22.25(14.15~34.80) 30.10(18.70~47.85) Z=-2.944 0.003 AST(U/L) 21.25(17.45~26.60) 23.70(19.65~35.20) Z=-2.555 0.011 ALP(U/L) 58.25(46.18~68.05) 60.90(51.20~74.05) Z=-1.616 0.106 GGT(U/L) 15.35(11.68~22.55) 18.10(14.20~28.70) Z=-2.466 0.014 UA(μmol/L) 287.5(244.8~345.3) 333.0(291.0~375.5) Z=-3.620 <0.001 TG(mmol/L) 0.77(0.62~1.02) 0.92(0.66~1.14) Z=-2.224 0.026 TC(mmol/L) 3.96±0.80 3.90±0.77 t=0.876 0.382 FBS(mmol/L) 4.28(4.05~4.58) 4.30(4.06~4.62) Z=-0.430 0.667 HDL(mmol/L) 1.35(1.10~1.56) 1.22(0.99~1.50) Z=-1.759 0.079 LDL(mmol/L) 2.27(1.83~2.65) 2.15(1.83~2.67) Z=-0.032 0.975 HBsAg(log10 IU/mL) 3.00(2.29~3.89) 3.30(2.87~4.06) Z=-1.774 0.076 HBeAg(+)[例(%)] 29(29.6) 29(35.8) χ2=0.781 0.377 HBV DNA(log10 IU/mL) 3.55(3.01~5.03) 4.31(3.01~7.06) Z=-1.692 0.091 CAP(dB/m)1) 218(201~237) 234(214~258) Z=-2.867 0.004 显著炎症[例(%)] 29(29.6) 28(34.6) χ2=0.506 0.477 显著纤维化[例(%)] 25(25.5) 35(43.2) χ2=6.234 0.013 注:FBS,空腹血糖。1)共102例数据参与分析,其中无脂肪变性组59例,极轻脂肪变性组43例。 表 2 不同BMI分组的基线特征
Table 2. Baseline clinical characteristics of the CHB patients, stratified by BMI
指标 正常体质量组(n=112) 超重组(n=57) 无脂肪变性组(n=69) 极轻脂肪变性组(n=43) 统计值 P值 无脂肪变性组(n=19) 极轻脂肪变性组(n=38) 统计值 P值 男[例(%)] 33(47.8) 28(65.1) χ2=3.193 0.074 11(57.9) 28(73.7) χ2=1.462 < 0.001 年龄(岁) 39.0(32.0~48.5) 37.0(29.0~47.0) Z=0.832 0.405 39.0(31.0~50.0) 36.0(32.8~48.5) Z=-0.356 0.722 高血压[例(%)] 1(1.4) 4(9.3) χ2=3.796 0.051 1(5.3) 1(2.6) χ2=0.255 0.614 PLT(×109/L) 161.0±44.7 164.0±48.5 t=-1.767 0.080 199.0±48.4 167.0±49.1 t=2.112 0.039 ALT(U/L) 22.1(14.1~31.5) 26.9(18.8~45.3) Z=-1.849 0.065 24.8(16.2~55.7) 34.2(18.6~55.9) Z=-1.10 0.271 AST(U/L) 21.3(17.4~26.0) 23.7(18.5~29.8) Z=-1.268 0.205 22.5(20.0~28.5) 23.9(19.9~41.2) Z=-1.067 0.286 ALP(U/L) 58.2(45.6~66.9) 60.9(51.6~69.2) Z=-1.161 0.246 60.8±19.7 62.3±19.5 t=0.285 0.776 GGT(U/L) 15.2(11.8~23.5) 16.4(14.1~21.1) Z=-1.182 0.237 18.5(12.8~23.3) 21.6(15.4~37.2) Z=-0.906 0.365 UA(μmol/L) 291.0±72.8 333.0±63.9 t=-2.395 0.018 340.0(254.0~397.0) 340.5(297.8~394.0) Z=-0.635 0.526 TG(mmol/L) 0.78(0.65~0.96) 0.87(0.62~1.13) Z=-1.364 0.172 1.04(0.69~1.34) 0.93(0.71~1.15) Z=-0.279 0.780 TC(mmol/L) 3.98(3.41~4.45) 3.25(3.19~4.36) Z=0.879 0.379 4.01±0.71 4.03±0.78 t=0.901 0.371 FBS(mmol/L) 4.22±0.39 4.29±0.44 t=-0.216 0.829 4.49(4.22~4.90) 4.33(4.03~4.66) Z=-1.168 0.243 HDL(mmol/L) 1.40±0.36 1.24±0.32 t=1.165 0.247 1.20(1.10~1.35) 1.21(0.93~1.48) Z=-0.330 0.741 LDL(mmol/L) 2.27(1.76~2.53) 2.06(1.73~2.54) Z=-0.835 0.404 2.61±0.61 2.30±0.49 t=1.106 0.314 HBsAg(log10 IU/mL) 2.99±1.17 3.30±1.04 t=-1.677 0.096 3.27±1.38 3.27±1.21 t=0.369 0.714 HBeAg(+)[例(%)] 19(27.5) 14(32.6) χ2=0.321 0.571 7(36.8) 15(39.5) χ2=0.037 0.847 HBV DNA(log10 IU/mL) 3.42(3.00~5.03) 4.44(3.51~6.79) Z=-2.474 0.013 3.74(3.26~7.53) 4.24(2.70~7.29) Z=-0.623 0.533 CAP(dB/m)1) 218.0(201.0~234.5) 217.0(206.0~243.5) Z=-0.570 0.569 237.0(218.0~246.0) 250.0(231.5~268.5) Z=-1.390 0.165 显著炎症[例(%)] 18(26.1) 12(27.9) χ2=0.045 0.832 6(31.6) 16(42.1) χ2=0.592 0.442 显著纤维化[例(%)] 20(29.0) 16(37.2) χ2=0.821 0.365 4(21.1) 19(50.0) χ2=4.410 0.036 注:1)4组对应的例数分别为41、22、11、21。 表 3 UA与肝组织学特征相关性分析
Table 3. Correlation between UA and liver histological features
组别 组织学特征 r值 P值 全部 炎症分级 0.004 0.969 纤维化分级 0.063 0.575 正常体质量组 炎症分级 -0.088 0.573 纤维化分级 0.136 0.384 超重组 炎症分级 0.024 0.884 纤维化分级 -0.055 0.744 表 4 CHB合并极轻脂肪变性危险因素的Logistic回归分析
Table 4. Univariate and multivariate analysis of risk factors for CHB complicated with minimal steatosis
变量 单因素 多因素 OR (95% CI) P值 OR (95% CI) P值 男性 2.431(1.313~4.500) 0.005 BMI 1.257(1.116~1.415) <0.001 1.223(1.086~1.378) 0.001 UA 1.007(1.003~1.011) 0.001 1.006(1.002~1.010) 0.008 TG 2.491(1.100~5.641) 0.029 HDL 0.426(0.176~1.032) 0.059 HBsAg 1.260(0.974~1.630) 0.078 HBV DNA < 3 log10 IU/mL 3~5 log10 IU/mL 0.650(0.302~1.400) 0.271 ≥5 log10 IU/mL 1.318(0.586~2.964) 0.504 表 5 正常体质量组CHB合并极轻脂肪变性危险因素的Logistic回归分析
Table 5. Univariate and multivariate analysis of risk factors for CHB complicated with minimal steatosis in normal BMI group
变量 单因素 多因素 OR(95%CI) P值 OR(95%CI) P值 男性 2.036(0.929~4.465) 0.076 UA 1.007(1.001~1.013) 0.022 1.007(1.001~1.013) 0.022 HBsAg 1.363(0.942~1.972) 0.100 HBV DNA <3 log10 IU/mL 3~5 log10 IU/mL 1.583(0.534~4.695) 0.407 ≥5 log10 IU/mL 2.833(0.909~8.834) 0.073 -
[1] LI J, ZOU B, YEO YH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis[J]. Lancet Gastroenterol Hepatol, 2019, 4(5): 389-398. DOI: 10.1016/S2468-1253(19)30039-1. [2] ZHENG Q, ZOU B, WU Y, et al. Systematic review with meta-analysis: prevalence of hepatic steatosis, fibrosis and associated factors in chronic hepatitis B[J]. Aliment Pharmacol Ther, 2021, 54(9): 1100-1109. DOI: 10.1111/apt.16595. [3] KLEINER DE, BRUNT EM, VAN NATTA M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease[J]. Hepatology, 2005, 41(6): 1313-1321. DOI: 10.1002/hep.20701. [4] WANG MM, WANG GS, SHEN F, et al. Hepatic steatosis is highly prevalent in hepatitis B patients and negatively associated with virological factors[J]. Dig Dis Sci, 2014, 59(10): 2571-2579. DOI: 10.1007/s10620-014-3180-9. [5] SCHEUER PJ. Classification of chronic viral hepatitis: a need for reassessment[J]. J Hepatol, 1991, 13(3): 372-374. DOI: 10.1016/0168-8278(91)90084-o. [6] LAI CL, RATZIU V, YUEN MF, et al. Viral hepatitis B[J]. The Lancet, 2003, 362(9401): 2089-2094. DOI: 10.1016/S0140-6736(03)15108-2. [7] SARIN SK, KUMAR M, LAU GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update[J]. Hepatol Int, 2016, 10(1): 1-98. DOI: 10.1007/s12072-015-9675-4. [8] LEE MH, YANG HI, LIU J, et al. Prediction models of long-term cirrhosis and hepatocellular carcinoma risk in chronic hepatitis B patients: risk scores integrating host and virus profiles[J]. Hepatology, 2013, 58(2): 546-554. DOI: 10.1002/hep.26385. [9] WONG GL, CHAN HL, YU Z, et al. Coincidental metabolic syndrome increases the risk of liver fibrosis progression in patients with chronic hepatitis B-a prospective cohort study with paired transient elastography examinations[J]. Aliment Pharmacol Ther, 2014, 39(8): 883-893. DOI: 10.1111/apt.12658. [10] YEN YH, CHANG KC, TSAI MC, et al. Elevated body mass index is a risk factor associated with possible liver cirrhosis across different etiologies of chronic liver disease[J]. J Formos Med Assoc, 2018, 117(4): 268-275. DOI: 10.1016/j.jfma.2017.09.002. [11] CHEN YC, HSU CW, JENG WJ, et al. Advanced liver fibrosis is associated with necroinflammatory grade but not hepatic steatosis in chronic hepatitis B patients[J]. Dig Dis Sci, 2021, 66(12): 4492-4500. DOI: 10.1007/s10620-020-06761-x. [12] SETO WK, FUNG J, CHEUNG KS, et al. Body-mass index is associated with fibrosis regression during long-term nucleoside analogue therapy in chronic hepatitis B[J]. Aliment Pharmacol Ther, 2016, 44(10): 1071-1079. DOI: 10.1111/apt.13804. [13] ZHU L, JIANG J, ZHAI X, et al. Hepatitis B virus infection and risk of non-alcoholic fatty liver disease: A population-based cohort study[J]. Liver Int, 2019, 39(1): 70-80. DOI: 10.1111/liv.13933. [14] PAIS R, RUSU E, ZILISTEANU D, et al. Prevalence of steatosis and insulin resistance in patients with chronic hepatitis B compared with chronic hepatitis C and non-alcoholic fatty liver disease[J]. Eur J Intern Med, 2015, 26(1): 30-36. DOI: 10.1016/j.ejim.2014.12.001. [15] NAU AL, SOARES JC, SHIOZAWA MB, et al. Clinical and laboratory characteristics associated with dyslipidemia and liver steatosis in chronic HBV carriers[J]. Rev Soc Bras Med Trop, 2014, 47(2): 158-164. DOI: 10.1590/0037-8682-0009-2014. [16] YILMAZ B, KOKLU S, BUYUKBAYRAM H, et al. Chronic hepatitis B associated with hepatic steatosis, insulin resistance, necroinflammation and fibrosis[J]. Afr Health Sci, 2015, 15(3): 714-718. DOI: 10.4314/ahs.v15i3.3. [17] CHEN Y, CAO D, LI C, et al. A nomogram for discrimination of non- alcoholic fatty liver disease in patients with chronic hepatitis B[J]. Eur J Gastroenterol Hepatol, 2021, 33(1): 69-75. DOI: 10.1097/MEG.0000000000001691. [18] YUAN H, YU C, LI X, et al. Serum uric acid levels and risk of metabolic syndrome: a dose-response meta-analysis of prospective studies[J]. J Clin Endocrinol Metab, 2015, 100(11): 4198-4207. DOI: 10.1210/jc.2015-2527. [19] HWANG IC, SUH SY, SUH AR, et al. The relationship between normal serum uric acid and nonalcoholic fatty liver disease[J]. J Korean Med Sci, 2011, 26(3): 386-391. DOI: 10.3346/jkms.2011.26.3.386. [20] XU CF, YU CH, XU L, et al. High serum uric acid increases the risk for nonalcoholic fatty liver disease: a prospective observational study[J]. PLoS One, 2010, 5(7): e11578. DOI: 10.1371/journal.pone.0011578. [21] ZHENG X, GONG L, LUO R, et al. Serum uric acid and non-alcoholic fatty liver disease in non-obesity Chinese adults[J]. Lipids Health Dis, 2017, 16(1): 202. DOI: 10.1186/s12944-017-0531-5. [22] ZHOU M, YANG N, XING X, et al. Obesity interacts with hyperuricemia on the severity of non-alcoholic fatty liver disease[J]. BMC Gastroenterol, 2021, 21(1): 43. DOI: 10.1186/s12876-021-01615-w. [23] PETTA S, CAMMÀ C, CABIBI D, et al. Hyperuricemia is associated with histological liver damage in patients with non-alcoholic fatty liver disease[J]. Aliment Pharmacol Ther, 2011, 34(7): 757-766. DOI: 10.1111/j.1365-2036.2011.04788.x. [24] FERNÁNDEZ RODRÍGUEZ CM, ALLER R, GUTIÉRREZ GARCÍA ML, et al. Higher levels of serum uric acid influences hepatic damage in patients with non-alcoholic fatty liver disease (NAFLD)[J]. Rev Esp Enferm Dig, 2019, 111(4): 264-269. DOI: 10.17235/reed.2019.5965/2018. [25] BALLESTRI S, NASCIMBENI F, ROMAGNOLI D, et al. The independent predictors of non-alcoholic steatohepatitis and its individual histological features. : Insulin resistance, serum uric acid, metabolic syndrome, alanine aminotransferase and serum total cholesterol are a clue to pathogenesis and candidate targets for treatment[J]. Hepatol Res, 2016, 46(11): 1074-1087. DOI: 10.1111/hepr.12656. [26] DUAN H, ZHANG R, CHEN X, et al. Associations of uric acid with liver steatosis and fibrosis applying vibration controlled transient elastography in the united states: a nationwide cross-section study[J]. Front Endocrinol (Lausanne), 2022, 13: 930224. DOI: 10.3389/fendo.2022.930224. -



 PDF下载 ( 2358 KB)
PDF下载 ( 2358 KB)


 下载:
下载: