12周索磷布韦联合可洛派韦治疗慢性丙型肝炎的效果和安全性分析
DOI: 10.3969/j.issn.1001-5256.2023.03.009
Efficacy and safety of the 12-week sofosbuvir-coblopasvir regimen in treatment of chronic hepatitis C
-
摘要:
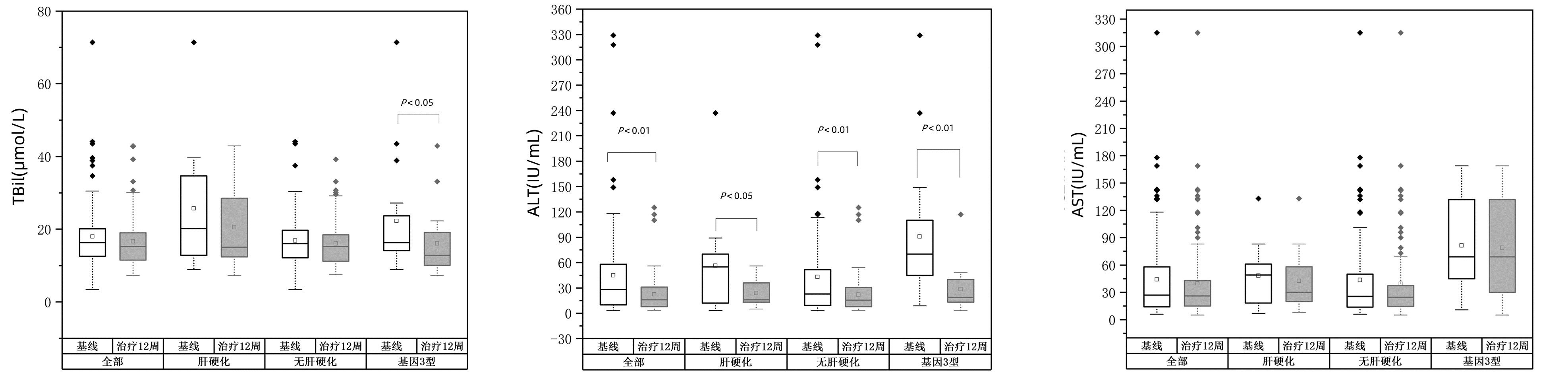
目的 分析索磷布韦联合盐酸可洛派韦12周方案治疗中国西北地区慢性丙型肝炎的效果和安全性。 方法 本研究纳入2021年7月1日—2021年12月31日空军军医大学第一附属医院、空军军医大学第二附属医院、西安交通大学第二附属医院、陕西省宝鸡市中心医院4家医院的慢性丙型肝炎(CHC)接受索磷布韦(400 mg) 联合盐酸可洛派韦(60 mg)治疗12周的101例患者,其中肝硬化13例,无肝硬化88例。无论是否有肝硬化、任何基因分型均未加用利巴韦林等其他抗病毒药物。提取患者基线、治疗12周及停药后12周的HCV RNA定量、肝生化指标等临床资料。主要评估治疗结束后12周持续病毒学应答(SVR12)和治疗12周时的安全性。其次评估治疗12周对肝生化指标的影响。不满足正态分布的计量资料采用M(P25~P75)表示,两组间比较采用Mann-Whitney U检验。 结果 共101例患者纳入分析,其中男性55例(54.5%),中位年龄53岁,12.8%有肝硬化,1.0%合并肝癌,3.0%为经治患者,3.0%合并有2型糖尿病。基因型分布:1型8%,2型60%,3型19%,6型6%,未检测基因型7%。101例患者经12周治疗HCV RNA均低于检测下限,SVR12达100%,与基线相比治疗12周血清ALT水平明显降低(P<0.05)。有阿托伐他汀钙、阿司匹林、二甲双胍、硝苯地平、双环醇、复方甘草酸苷等合并用药的患者为22.7%。任何不良事件发生率为16.8%,疲乏(12.9%)最常见。 结论 12周索磷布韦联合盐酸可洛派韦治疗西北地区CHC患者可获得较高的SVR12,抗病毒治疗安全性良好,治疗12周时患者血清ALT异常明显改善。 Abstract:Objective To investigate the efficacy and safety of the 12-week regimen with sofosbuvir and coblopasvir hydrochloride in the treatment of chronic hepatitis C (CHC) in northwest China. Methods This study enrolled 101 patients with CHC of any genotype who received sofosbuvir (400 mg) combined with coblopasvir hydrochloride (60 mg) for 12 weeks in The First Affiliated Hospital of Air Force Medical University, The Second Affiliated Hospital of Air Force Medical University, The Second Affiliated Hospital of Xi'an Jiaotong University, and Baoji Central Hospital from July 1 to December 31, 2021, among whom 13 had liver cirrhosis and 88 did not have live cirrhosis. Other antiviral drugs such as ribavirin were not added regardless of the presence or absence of liver cirrhosis or the genotype of CHC. Related clinical data ere extracted, including HCV RNA quantification and liver biochemical parameters at baseline, at week 12 of treatment, and at 12 weeks after drug withdrawal. The primary endpoints were sustained virologic response at 12 weeks after the end of treatment (SVR12) and safety at week 12 of treatment, and the secondary endpoint was the effect of the 12-week treatment on liver biochemical parameters. The non-normally distributed continuous data were expressed as M(P25-P75), and the Mann-Whitney U test was used for comparison between groups. Results A total of 101 patients were included in the analysis, among whom there were 55 male patients (54.5%) and 46 female patients, and the median age was 53 years. Among these patients, 12.8% had liver cirrhosis, 1.0% had liver cancer, 3.0% were treatment-experienced patients, and 3.0% had type 2 diabetes. As for genotype distribution, 8% had CHC genotype 1, 60% had CHC genotype 2, 19% had CHC genotype 3, and 6% had CHC genotype 6, and genotype was not tested for 7% of the patients. After 12 weeks of treatment, all 101 patients had a HCV RNA level of below the lower limit of detection and an SVR12 rate of 100%, with a significant reduction in the serum level of alanine aminotransferase (ALT) from baseline to week 12 of treatment (P < 0.05). Among these patients, 22.7% had concomitant medications such as atorvastatin calcium, aspirin, metformin, nifedipine, bicyclol, and compound glycyrrhizin. The incidence rate of adverse events was 16.8%, and fatigue (12.9%) was the most common adverse event. Conclusion The 12-week treatment with sofosbuvir and coblopasvir hydrochloride can obtain high SVR12 in CHC patients in northwest China and has good antiviral safety, with a significant improvement in abnormal serum ALT at week 12 of treatment. -
Key words:
- Hepatitis C, Chronic /
- Coblopasvir /
- Sofosbuvir /
- Treatment Outcome
-
表 1 患者基线临床特点
Table 1. Baseline clinical characteristics of patients
指标 总体(n=101) 肝硬化(n=13) 无肝硬化(n=88) 男性[例(%)] 55(54.5) 9(69.2) 47(53.4) 年龄(岁) 53(45.0~59.5) 54(44.0~59.5) 53(45.0~59.8) ≥60岁[例(%)] 24(23.8) 3(23.1) 21(23.9) TBil(μmol/L) 16.3(12.3~20.2) 20.2(12.4~36.8) 16.1(11.9~19.7) ALT(U/L) 28(9.8~58.5) 55(11.7~74.0) 23(9.3~52.3) AST(U/L) 27.0(13.9~58.5) 49.0(15.8~63.0) 25.5(13.9~50.5) HCV RNA(log10 IU/mL) 6(5.0~6.0) 5(4.5~6.0) 6(5.0~6.0) eGFR (mL·min-1·1.73 m-2) 111.5(94.6~120.0) 113.9(92.2~121.3) 111.0(96.8~120.0) 表 2 治疗前后生化指标变化
Table 2. Changes of biochemical indexes before and after treatment
组别 基线 治疗12周 Z值 P值 TBil(μmol/L) 全部(n=101) 16.3(12.3~20.2) 15.2(11.4~19.1) -1.648 0.990 肝硬化(n=13) 20.2(12.4~36.8) 15.0(12.2~29.3) -0.785 0.433 无肝硬化(n=88) 16.1(11.9~19.7) 15.2(11.2~18.6) -1.408 0.159 基因3型(n=19) 16.3(8.0~30.8) 16.3(14.1~23.7) -2.616 0.009 ALT(U/L) 全部(n=101) 28.0(9.8~58.5) 16.0(8.2~31.0) -5.506 <0.001 肝硬化(n=13) 55.0(11.7~74.0) 16.2(12.0~38.0) -2.482 0.013 无肝硬化(n=88) 23.0(9.3~52.3) 15.5(8.0~30.8) -4.937 <0.001 基因3型(n=19) 70.0(41.2~110.0) 19.0(13.0~40.0) -3.703 <0.001 AST(U/L) 全部(n=101) 27.0(13.9~58.5) 16.2(12.0~38.0) -1.944 0.052 肝硬化(n=13) 49.0(15.8~63.0) 30.0(17.2~59.5) -1.682 0.092 无肝硬化(n=88) 25.5(13.9~50.5) 24.5(14.2~37.8) -1.312 0.189 基因3型(n=19) 69.0(45.0~132.0) 69.0(30.0~132.0) -1.095 0.273 表 3 治疗前后eGFR变化
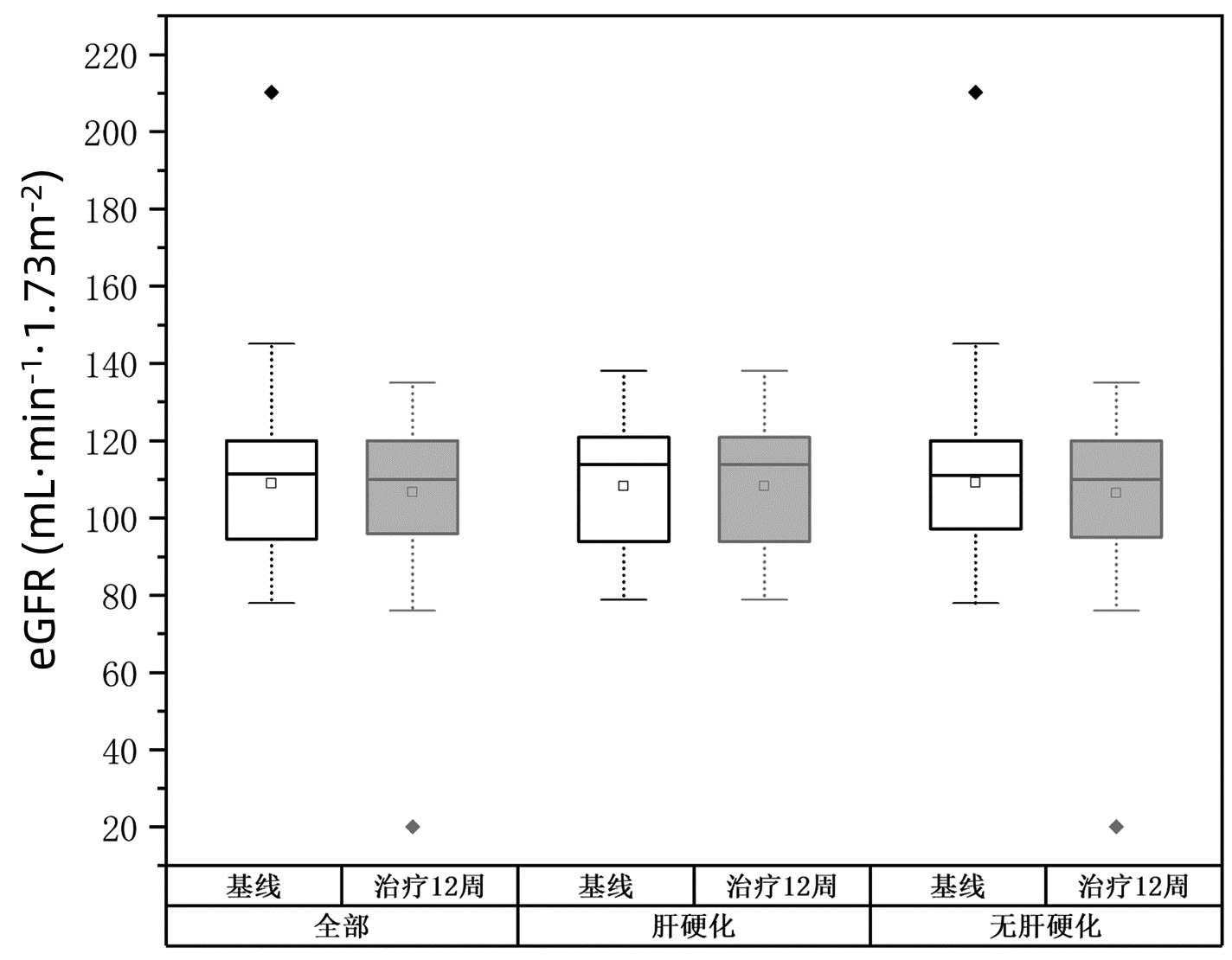
Table 3. Changes of eGFR before and after treatment
组别 例数 eGFR(mL·min-1·1.73 m-2) Z值 P值 基线 治疗12周 全部 101 111.5(94.6~120.0) 110.0(95.4~120.0) -0.545 0.586 肝硬化 13 113.9(92.2~121.3) 113.8(94.1~120.0) -0.454 0.650 无肝硬化 88 111.0(96.8~120.0) 109.8(95.4~120.0) -0.376 0.707 基因3型 19 120.0(109.6~120.0) 117.9(101.4~120.0) -1.655 0.098 表 4 盐酸可洛派韦/索磷布韦不良事件
Table 4. Adverse events of Sofosbuvir/Coblopasvir
不良反应/事件 总体
(n=101)肝硬化
(n=13)无肝硬化
(n=88)全部不良事件[例(%)] 17(16.6) 5(38.5) 12(13.6) 严重不良事件[例(%)] 0 0 0 乏力[例(%)] 13(12.9) 4(30.8) 9(10.2) 恶心[例(%)] 1(1) 0 1(1.1) 失眠[例(%)] 2(1.98) 1(7.7) 1(1.1) 食欲下降[例(%)] 1(1) 0 1(1.1) -
[1] EL-SERAG HB. Epidemiology of viral hepatitis and hepatocellular carcinoma[J]. Gastroenterology, 2012, 142(6): 1264-1273. e1. DOI: 10.1053/j.gastro.2011.12.061. [2] Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study[J]. Lancet Gastroenterol Hepatol, 2017, 2(3): 161-176. DOI: 10.1016/S2468-1253(16)30181-9. [3] KHAN H, PAESHUYSE J, MURAD S, et al. Assessment of the activity of directly acting antivirals and other products against different genotypes of hepatitis C virus prevalent in resource-poor countries[J]. Antiviral Res, 2016, 125: 43-45. DOI: 10.1016/j.antiviral.2015.10.008. [4] GAO LH, NIE QH, ZHAO XT. Drug-drug interactions of newly approved direct-acting antiviral agents in patients with hepatitis C[J]. Int J Gen Med, 2021, 14: 289-301. DOI: 10.2147/IJGM.S283910. [5] FU Z, DONG C, GE Z, et al. High SVR12 with 8-week course of direct-acting antivirals in adolescents and children with chronic hepatitis C: A comprehensive analysis[J]. Front Med (Lausanne), 2021, 8: 608760. DOI: 10.3389/fmed.2021.608760. [6] Chinese Society of Hepatology, Chinese Medical Association, Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of hepatitis C(2019 version)[J]. J Clin Hepatol, 2019, 35(12): 2670-2686. DOI: 10.3969/j.issn.1001-5256.2019.12.008.中华医学会肝病学分会, 中华医学会感染病学分会. 丙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35(12): 2670-2686. DOI: 10.3969/j.issn.1001-5256.2019.12.008. [7] RAO H, LIU H, WU E, et al. Comparison of clinical outcomes and impact of SVR in American and Chinese patients with chronic hepatitis C[J]. JHEP Rep, 2020, 2(4): 100136. DOI: 10.1016/j.jhepr.2020.100136. [8] LI C, LI X, ZHU X, et al. Pharmacokinetics, safety, and tolerability of ledipasvir/sofosbuvir and sofosbuvir/velpatasvir in healthy chinese subjects[J]. Clin Ther, 2020, 42(3): 448-457. DOI: 10.1016/j.clinthera.2020.01.013. [9] LI J, LI G, WANG J, et al. Efficacy and safety of elbasvir/grazoprevir treatment for Chinese patients with hepatitis C virus genotype 1b: a retrospective study[J]. Am J Transl Res, 2022, 14(6): 3995-4005. [10] HUANG CF, ⅡO E, JUN DW, et al. Direct-acting antivirals in East Asian hepatitis C patients: real-world experience from the REAL-C Consortium[J]. Hepatol Int, 2019, 13(5): 587-598. DOI: 10.1007/s12072-019-09974-z. [11] HUANG K, CHEN J, XU R, et al. Molecular evolution of hepatitis C virus in China: A nationwide study[J]. Virology, 2018, 516: 210-218. DOI: 10.1016/j.virol.2018.01.015. [12] PIECHA F, GÄNßLER JM, OZGA AK, et al. Treatment and re-treatment results of HCV patients in the DAA era[J]. PLoS One, 2020, 15(5): e0232773. DOI: 10.1371/journal.pone.0232773. [13] RAO H, WEI L, LOPEZ-TALAVERA JC, et al. Distribution and clinical correlates of viral and host genotypes in Chinese patients with chronic hepatitis C virus infection[J]. J Gastroenterol Hepatol, 2014, 29(3): 545-553. DOI: 10.1111/jgh.12398. [14] CHEN Y, YU C, YIN X, et al. Hepatitis C virus genotypes and subtypes circulating in Mainland China[J]. Emerg Microbes Infect, 2017, 6(11): e95. DOI: 10.1038/emi.2017.77. [15] WU N, RAO HY, YANG WB, et al. Impact of hepatitis C virus genotype 3 on liver disease progression in a Chinese national cohort[J]. Chin Med J (Engl), 2020, 133(3): 253-261. DOI: 10.1097/CM9.0000000000000629. [16] LU J, FENG Y, CHEN L, et al. Subtype-specific prevalence of hepatitis C virus NS5A resistance associated substitutions in Mainland China[J]. Front Microbiol, 2019. DOI: 10.3389/fmicb.2019.00535. [17] CHEN YS, HUANG KH, WANG PM, et al. The impact of direct-acting antiviral therapy on the risk of recurrence after curative resection in patients with hepatitis-C-virus-related early stage hepatocellular carcinoma[J]. Medicina (Kaunas), 2022, 58(2): 259. DOI: 10.3390/medicina58020259. [18] TANAKA S, SHINKAWA H, TAMORI A, et al. Surgical outcomes for hepatocellular carcinoma detected after hepatitis C virus eradiation by direct-acting antivirals[J]. J Surg Oncol, 2020, 122(8): 1543-1552. DOI: 10.1002/jso.26184. [19] KUO YH, WANG JH, CHANG KC, et al. The influence of direct-acting antivirals in hepatitis C virus related hepatocellular carcinoma after curative treatment[J]. Invest New Drugs, 2020, 38(1): 202-210. DOI: 10.1007/s10637-019-00870-9. [20] MONTALDO C, TERRI M, RICCIONI V, et al. Fibrogenic signals persist in DAA-treated HCV patients after sustained virological response[J]. J Hepatol, 2021, 75(6): 1301-1311. DOI: 10.1016/j.jhep.2021.07.003. [21] XIA H, ZHANG Y, ZAONGO SD, et al Direct-acting antiviral treatments display excellent outcomes even in older HCV-infected patients at increased risk of fibrosis[J]. Ann Transl Med, 2021, 9(10): 847. DOI: 10.21037/atm-21-1297. [22] KANDA T, LAU GKK, WEI L, et al. APASL clinical practice recommendation: how to treat HCV-infected patients with renal impairment?[J]. Hepatol Int, 2019, 13(2): 103-109. DOI: 10.1007/s12072-018-9915-5. -



 PDF下载 ( 2213 KB)
PDF下载 ( 2213 KB)


 下载:
下载: