-
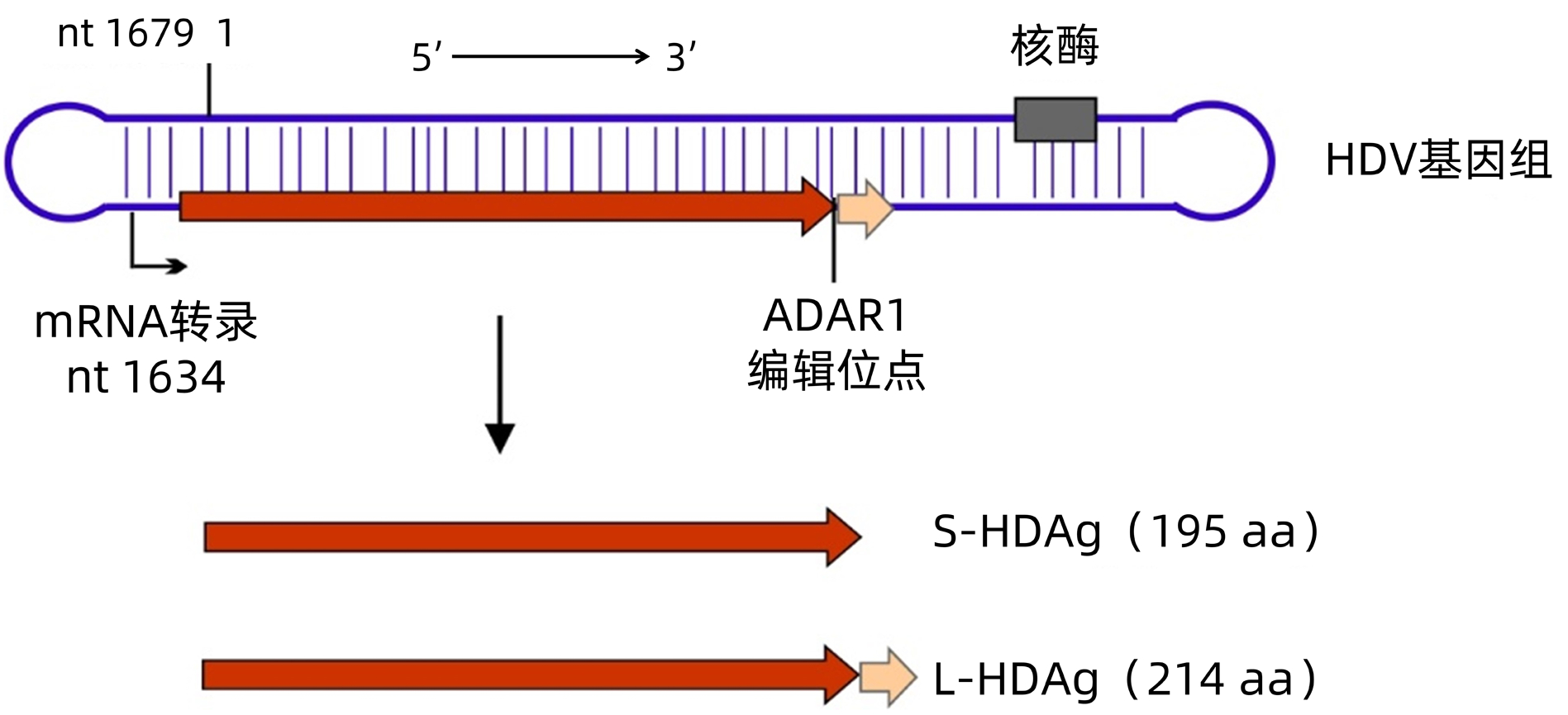
摘要: 丁型肝炎是由丁型肝炎病毒(HDV)与乙型肝炎病毒(HBV)合并感染或者HDV重叠感染HBV携带者引起的一种严重病毒性肝炎。由于长期重视不足,丁型肝炎的诊断目前还存在巨大空白。近年来,随着相关研究的不断深入,科研和医疗行业逐渐意识到丁型肝炎的危害。同时相关药物研发的重大进展也为丁型肝炎的治疗甚至治愈带来了新的机遇。而这些进展将大大增加对丁型肝炎诊断的需求。抗-HD抗体是丁型肝炎诊断的关键指标。本文就目前丁型肝炎抗体包括抗-HD总抗体、IgG和IgM的检测方法进行了总结和比较分析,并对相关的重要问题进行了讨论,以期加深对丁型肝炎抗体检测现状的了解,并为开发更好的丁型肝炎诊断工具提供参考。Abstract: Hepatitis D is a severe form of viral hepatitis caused by co-infection with hepatitis D virus (HDV) and hepatitis B virus (HBV) or superinfection of HDV in HBV carriers. There is still a huge gap in the diagnosis of hepatitis D due to insufficient emphasis on this disease for a long time. With the advances in related studies in recent years, the academia and the medical industry have gradually realized the harm of hepatitis D, and meanwhile, breakthroughs in drug development have also brought new opportunities for the treatment or even cure of hepatitis D. These advances greatly increase the demand for the diagnosis of hepatitis D. HDV antibodies are the key markers for the diagnosis of hepatitis D. This article summarizes and compares the detection methods for HDV antibodies including total HDV antibodies, IgG, and IgM and discusses related important issues, so as to understand the current status of the detection of HDV antibodies and provide a reference for developing better diagnostic tools for hepatitis D.
-
Key words:
- Hepatitis Delta Virus /
- Hepatitis D /
- Hepatitis Antibodies /
- Diagnosis
-
表 1 不同感染状态下丁型肝炎的血清抗体
Table 1. Serum hepatitis D antibodies in different infection stages
感染类型 HDV感染阶段 抗-HD IgM 抗-HD IgG 抗-HBc IgM HBsAg HDV/HBV合并感染 急性期 阳性 阴性 阳性 阳性 转为慢性感染(<5%) 阴性或阳性 阳性 阴性 阳性 HDV/HBV被清除(>95%) 阴性 阳性或阴性 阴性 阴性 HDV/HBV重叠感染 急性期 阳性 阴性 阴性 阳性(下降) 转为慢性感染(>90%) 阴性或阳性 阳性 阴性 阳性 HDV被清除 阴性 阳性或阴性 阴性 阳性 表 2 丁型肝炎抗体检测方法
Table 2. Methods to measure hepatitis D antibodies
检测方法 抗HDV类型 检测方法 优点 缺点 产品 ELISA IgM 间接法 操作简便 受IgG干扰 很少 捕获法 无IgG干扰,重复性好 相对于间接法而言,检测略复杂 较多 IgG 间接法 操作简便 酶标二抗可能存在非特异反应 很多 总抗体 双抗原夹心法 检测灵敏度和特异性高 需要对抗原进行标记,成本高 很少 竞争法 适合复杂样品,重复性好 要求抗原表位少 很少 RIA 同ELISA 同ELISA 灵敏度非常高 放射性危害大 已退市 CLIA 总抗体 竞争法 灵敏度非常高 设备普及率较低 很少 试纸条 IgG 胶体金标记,类似于间接法 现场快速检测,不需要大型仪器 灵敏度较低 未上市 微阵列 IgG 荧光标记,类似于间接法 灵敏度非常高,适合高通量 技术和设备普及率低 未上市 -
[1] RIZZETTO M, HAMID S, NEGRO F. The changing context of hepatitis D[J]. J Hepatol, 2021, 74(5): 1200-1211. DOI: 10.1016/j.jhep.2021.01.014. [2] PAPATHEODORIDI M, PAPATHEODORIDIS GV. Is hepatitis delta underestimated?[J]. Liver Int, 2021, 41(Suppl 1): 38-44. DOI: 10.1111/liv.14833. [3] STOCKDALE AJ, KREUELS B, HENRION M, et al. The global prevalence of hepatitis D virus infection: Systematic review and meta-analysis[J]. J Hepatol, 2020, 73(3): 523-532. DOI: 10.1016/j.jhep.2020.04.008. [4] CHEN HY, SHEN DT, JI DZ, et al. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis[J]. Gut, 2019, 68(3): 512-521. DOI: 10.1136/gutjnl-2018-316601. [5] MIAO Z, ZHANG S, OU X, et al. Estimating the global prevalence, disease progression, and clinical outcome of hepatitis delta virus infection[J]. J Infect Dis, 2020, 221(10): 1677-1687. DOI: 10.1093/infdis/jiz633. [6] URBAN S, NEUMANN-HAEFELIN C, LAMPERTICO P. Hepatitis D virus in 2021: virology, immunology and new treatment approaches for a difficult-to-treat disease[J]. Gut, 2021, 70(9): 1782-1794. DOI: 10.1136/gutjnl-2020-323888. [7] SARIN SK, KUMAR M, LAU GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update[J]. Hepatol Int, 2016, 10(1): 1-98. DOI: 10.1007/s12072-015-9675-4. [8] European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection[J]. J Hepatol, 2017, 67(2): 370-398. DOI: 10.1016/j.jhep.2017.03.021. [9] LUCIFORA J, DELPHIN M. Current knowledge on hepatitis delta virus replication[J]. Antiviral Res, 2020, 179: 104812. DOI: 10.1016/j.antiviral.2020.104812. [10] LANGE M, ZARET D, KUSHNER T. Hepatitis delta: Current knowledge and future directions[J]. Gastroenterol Hepatol (N Y), 2022, 18(9): 508-520. [11] DÉNY P. Hepatitis delta virus genetic variability: from genotypes Ⅰ, Ⅱ, Ⅲ to eight major clades?[J]. Curr Top Microbiol Immunol, 2006, 307: 151-171. DOI: 10.1007/3-540-29802-9_8. [12] LE GAL F, BRICHLER S, DRUGAN T, et al. Genetic diversity and worldwide distribution of the deltavirus genus: A study of 2, 152 clinical strains[J]. Hepatology, 2017, 66(6): 1826-1841. DOI: 10.1002/hep.29574. [13] YURDAYD1N C, IDILMAN R, BOZKAYA H, et al. Natural history and treatment of chronic delta hepatitis[J]. J Viral Hepat, 2010, 17(11): 749-756. DOI: 10.1111/j.1365-2893.2010.01353.x. [14] FARCI P, NIRO GA. Clinical features of hepatitis D[J]. Semin Liver Dis, 2012, 32(3): 228-236. DOI: 10.1055/s-0032-1323628. [15] BRICHLER S, LE GAL F, NERI-PINTO F, et al. Serological and molecular diagnosis of hepatitis delta virus infection: results of a French national quality control study[J]. J Clin Microbiol, 2014, 52(5): 1694-1697. DOI: 10.1128/JCM.03521-13. [16] RICCO G, POPA DC, CAVALLONE D, et al. Quantification of serum markers of hepatitis B (HBV) and Delta virus (HDV) infections in patients with chronic HDV infection[J]. J Viral Hepat, 2018, 25(8): 911-919. DOI: 10.1111/jvh.12895. [17] WRANKE A, HEIDRICH B, ERNST S, et al. Anti-HDV IgM as a marker of disease activity in hepatitis delta[J]. PLoS One, 2014, 9(7): e101002. DOI: 10.1371/journal.pone.0101002. [18] GOWANS EJ, MACNAUGHTON TB, MICKAN L, et al. Use of recombinant hepatitis delta antigen in diagnostic assays for HDV antibody[J]. J Virol Methods, 1990, 27(1): 69-78. DOI: 10.1016/0166-0934(90)90147-8. [19] TSWANA SA, LAHER S. Detection of anti-delta antibodies among acute hepatitis B virus-infected patients[J]. J Med Virol, 1988, 25(4): 471-474. DOI: 10.1002/jmv.1890250410. [20] BEZEAUD A, ROSENSWAJG M, GUILLIN MC. Evaluation of five hepatitis delta virus marker assays for detection of antigen and antibody[J]. J Clin Microbiol, 1989, 27(12): 2880. DOI: 10.1128/jcm.27.12.2880-.1989. [21] SHATTOCK AG, MORRIS MC. Evaluation of commercial enzyme immunoassays for detection of hepatitis delta antigen and anti-hepatitis delta virus (HDV) and immunoglobulin M anti-HDV antibodies[J]. J Clin Microbiol, 1991, 29(9): 1873-1876. DOI: 10.1128/jcm.29.9.1873-1876.1991. [22] KUO YB, CHAO M, LEE YH, et al. New enzyme-linked immunosorbent assay for detection of antibodies against hepatitis delta virus using a hepatitis delta antigen derived from a Taiwanese clone and comparison to the Abbott radioimmunoassay[J]. Clin Vaccine Immunol, 2012, 19(5): 817-819. DOI: 10.1128/CVI.05687-11. [23] GOVINDARAJAN S, VALINLUCK B, LAKE-BAKKAR G. Evaluation of a commercial anti-delta EIA kit for detection of antibodies to hepatitis delta virus[J]. Am J Clin Pathol, 1991, 95(2): 240-241. DOI: 10.1093/ajcp/95.2.240. [24] DE ORY F, MINGUITO T, BALFAGÓN P, et al. Comparison of chemiluminescent immunoassay and ELISA for measles IgG and IgM[J]. APMIS, 2015, 123(8): 648-651. DOI: 10.1111/apm.12413. [25] LEMPP FA, ROGGENBACH I, NKONGOLO S, et al. A rapid point-of-care test for the serodiagnosis of hepatitis delta virus infection[J]. Viruses, 2021, 13(12): 2371. DOI: 10.3390/v13122371. [26] ROGGENBACH I, CHI X, LEMPP FA, et al. HDV seroprevalence in HBsAg-positive patients in China occurs in hotspots and is not associated with HCV mono-infection[J]. Viruses, 2021, 13(9): 1799. DOI: 10.3390/v13091799. [27] CHEN X, OIDOVSAMBUU O, LIU P, et al. A novel quantitative microarray antibody capture assay identifies an extremely high hepatitis delta virus prevalence among hepatitis B virus-infected mongolians[J]. Hepatology, 2017, 66(6): 1739-1749. DOI: 10.1002/hep.28957. [28] LU XX, YI Y, SU QD, et al. Expression and purification of recombinant hepatitis delta virus (HDV) antigen for use in a diagnostic ELISA for HDV infection using the high-density fermentation strategy in Escherichia coli[J]. Biomed Environ Sci, 2016, 29(6): 417-423. DOI: 10.3967/bes2016.054. [29] WANG W, LEMPP FA, SCHLUND F, et al. Assembly and infection efficacy of hepatitis B virus surface protein exchanges in 8 hepatitis D virus genotype isolates[J]. J Hepatol, 2021, 75(2): 311-323. DOI: 10.1016/j.jhep.2021.03.025. [30] COLLER KE, BUTLER EK, LUK KC, et al. Development and performance of prototype serologic and molecular tests for hepatitis delta infection[J]. Sci Rep, 2018, 8(1): 2095. DOI: 10.1038/s41598-018-20455-5. [31] CHOW SK, ATIENZA EE, COOK L, et al. Comparison of enzyme immunoassays for detection of antibodies to hepatitis D virus in serum[J]. Clin Vaccine Immunol, 2016, 23(8): 732-734. DOI: 10.1128/CVI.00028-16. [32] LE GAL F, BRICHLER S, SAHLI R, et al. First international external quality assessment for hepatitis delta virus RNA quantification in plasma[J]. Hepatology, 2016, 64(5): 1483-1494. DOI: 10.1002/hep.28772. [33] ZHANG ZF, ZHUANG H. Research progress on laboratory diagnosis of hepatitis D[J]. Chin J Viral Dis, 2021, 11(6): 407-413. DOI: 10.16505/j.2095-0136.2021.0052.张振锋, 庄辉. 丁型肝炎实验室诊断研究进展[J]. 中国病毒病杂志, 2021, 11(6): 407-413. DOI: 10.16505/j.2095-0136.2021.0052. -



 PDF下载 ( 1903 KB)
PDF下载 ( 1903 KB)

 下载:
下载:


