甲胎蛋白应答评价索拉非尼联合卡瑞利珠单抗治疗晚期肝细胞癌的效果分析
DOI: 10.3969/j.issn.1001-5256.2023.04.015
Value of alpha-fetoprotein response in evaluating the efficacy of sorafenib combined with camrelizumab in treatment of advanced hepatocellular carcinoma
-
摘要:
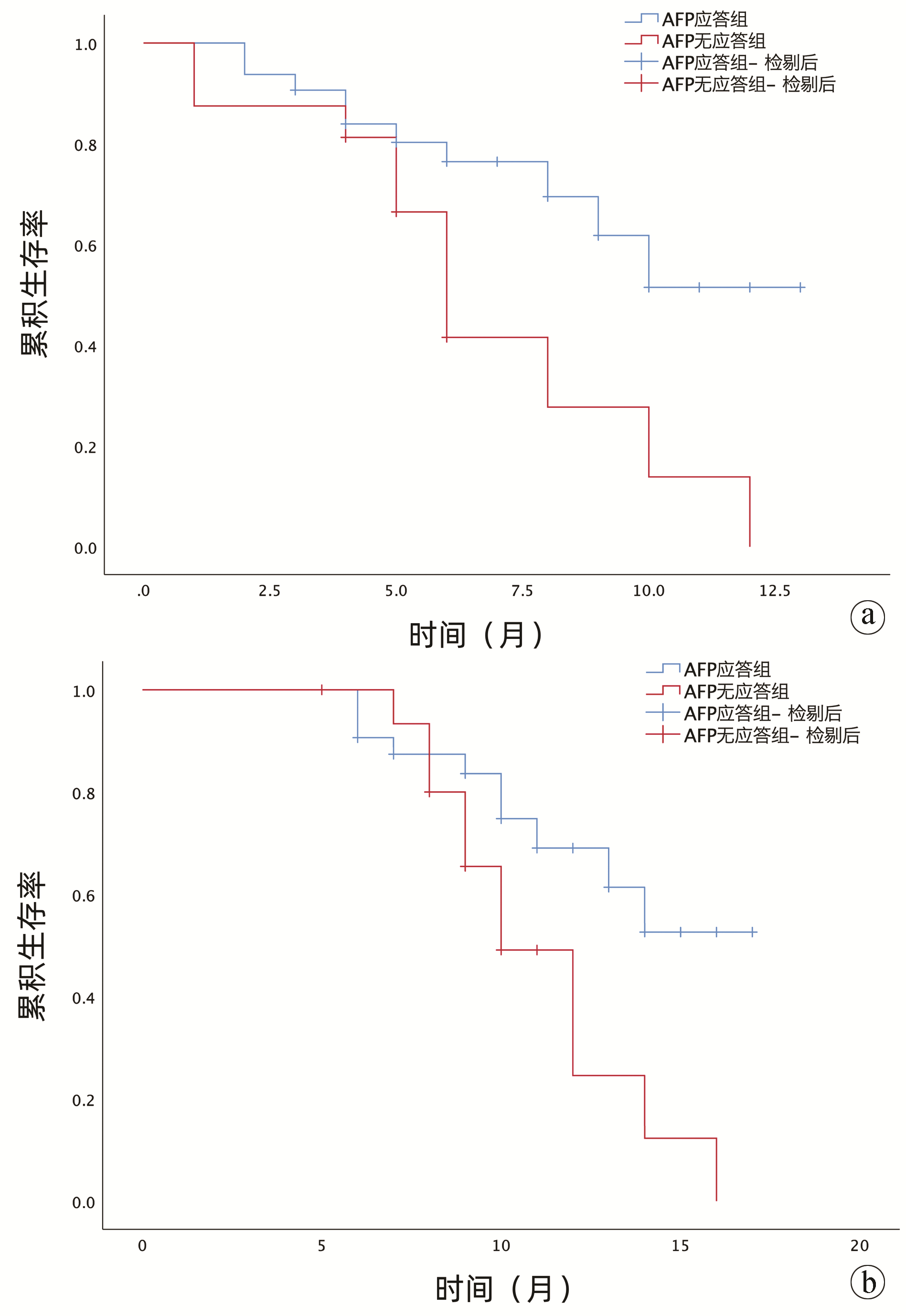
目的 探讨甲胎蛋白(AFP)应答在索拉非尼联合卡瑞利珠单抗治疗晚期肝细胞癌中的临床疗效及安全性。 方法 选取2020年9月—2022年2月于新疆医科大学第一附属医院接受索拉非尼联合卡瑞利珠单抗治疗的48例晚期肝细胞癌患者的临床资料,按照治疗后AFP应答水平进行分组:应答组(治疗6~8个月时,与基础AFP比较,AFP下降率>20%,n=32);无应答组(治疗6~8个月时,与基础AFP比较,AFP下降率≤20%, n=16)。计量资料组间比较采用Mann-Whitney U检验;计数资料组间比较采用χ2检验。绘制生存曲线,行Cox单因素/多因素回归分析得到与OS相关的独立危险因素。比较两组患者无进展生存时间、总生存期与治疗效果。 结果 两组患者中均未观察到达到临床缓解的病例。AFP应答组的客观有效率(21.88% vs 0)和疾病控制率(84.38% vs 43.75%) 均高于无应答组(χ2值分别为2.530、6.668,P值分别为0.112、0.010)。应答组无进展生存期(9.9个月)、总生存期(13.8个月)均高于无应答组(6.8个月、11.1个月)。AFP早期无应答(HR=2.624,95%CI:1.097~6.277,P=0.030)及肝外转移(HR=0.392,95%CI:0.157~0.978,P=0.045)与较短的无进展生存期独立相关。未出现因不良反应导致停药事件的发生。 结论 AFP早期应答在预测索拉非尼联合卡瑞利珠单抗治疗晚期肝细胞癌患者疗效及预后方面具有较高的临床价值。 Abstract:Objective To investigate the value of alpha-fetoprotein (AFP) response in evaluating the clinical efficacy and safety of sorafenib combined with camrelizumab in the treatment of advanced hepatocellular carcinoma (HCC). Methods Clinical data were collected from 48 patients with advanced HCC who were treated with sorafenib combined with camrelizumab in The First Affiliated Hospital of Xinjiang Medical University from September 2020 to February 2022, and according to the level of AFP response after treatment, they were divided into response group with 32 patients (AFP after 6-8 months of treatment was reduced by more than 20% compared with baseline AFP) and non-response group with 16 patients (AFP after 6-8 months of treatment was reduced by less than 20% compared with baseline AFP). The Mann-Whitney U test was used for comparison of continuous data between groups, and the chi-square test was used for comparison of categorical data between groups. Survival curves were plotted, and univariate and multivariate Cox regression analyses were used to investigate the independent risk factors for overall survival (OS). Progression free survival (PFS) time, OS time, and treatment outcome were compared between the two groups. Results No patient achieved clinical remission in either group. Compared with the non-response group, the response group had significantly higher objective response rate (21.88% vs 0, χ2=2.530, P=0.112) and disease control rate (84.38% vs 43.75%, χ2=6.668, P=0.010). Compared with the non-response group, the response group had longer PFS time (9.9 months vs 6.8 months) and OS time (13.8 months vs 11.1 months). Early non-response of AFP (hazard ratio [HR]=2.624, 95% confidence interval [CI]: 1.097-6.277, P=0.030) and extrahepatic metastasis (HR=0.392, 95%CI: 0.157-0.978, P=0.045) were independently associated with a shorter PFS time. No adverse event leading to drug withdrawal was observed in the study. Conclusion Early AFP response has a high clinical value in predicting the efficacy of sorafenib combined with camrelizumab in the treatment of advanced HCC and the prognosis of such patients. -
Key words:
- Carcinoma, Hepatocellular /
- alpha-Fetoproteins /
- Sorafenib
-
表 1 基线特征的比较
Table 1. Comparison of baseline characteristics
变量 应答组(n=32) 无应答组(n=16) 统计值 P值 性别[例(%)] χ2=1.497 0.239 男 23(71.80) 15(93.80) 女 9(28.00) 1(6.30) 年龄[例(%)] χ2=0.381 0.573 ≥60岁 13(40.60) 8(50.00) <60岁 19(59.40) 8(50.00) 基线AFP(ng/mL) 353.31(113.10~1 015.87) 1 616.89(92.08~2 000.00) Z=-2.203 0.088 AST(U/L) 49.10(37.26~97.53) 63.56(42.36~91.18) Z=-0.787 0.331 ALT(U/L) 48.89(36.42~68.00) 39.73(30.73~68.11) Z=-0.437 0.662 TBil(mol/L) 20.85(17.51~30.61) 17.69(15.46~19.48) Z=-3.040 0.002 Alb(g/L) 38.59(31.32~41.34) 36.19(33.53~37.88) Z=-0.262 0.793 PLT(×109/L) 112.00(60.25~163.25) 141.50(100.50~191.25) Z=-1.488 0.137 Hb(g/L) 138.00(125.25~147.75) 123.50(107.50~146.00) Z=-1.969 0.049 PT(s) 13.20(12.60~14.50) 13.20(12.00~13.85) Z=-0.941 0.347 INR 1.16(1.11~1.25) 1.15(1.06~1.20) Z=-0.733 0.463 病因[例(%)] χ2=0.034 0.854 HBV 30(93.80) 14(87.50) HCV 2(6.30) 2(12.50) ECOG评分[例(%)] χ2=0.671 0.413 0 16(50.0) 6(37.50) 1 16(50.0) 10(62.50) 肝硬化[例(%)] 26(81.25) 15(93.75) χ2=0.523 0.470 腹水[例(%)] 27(84.38) 14(87.50) χ2=0.000 1.000 远处转移[例(%)] 7(21.88) 5(68.75) χ2=0.125 0.480 门静脉癌栓[例(%)] 8(25.00) 8(25.00) χ2=3.000 0.083 表 2 AFP早期应答组和无应答组的临床疗效比较
Table 2. Comparison of clinical outcomes between the early responders and non-responders of AFP
临床疗效 应答组(n=32) 无应答组(n=16) CR(例) 0 0 PR(例) 7 0 SD(例) 20 7 PD(例) 5 9 ORR 21.88% 0 DCR 84.38% 43.75% 表 3 PFS相关的单因素和多因素分析
Table 3. PFS-related univariate and multifactorial analyses
变量 单因素分析 多因素分析 HR(95%CI) P值 HR(95%CI) P值 年龄(≥60岁vs<60岁) 1.152(0.479~2.771) 0.751 AFP(早期无应答vs有应答) 2.751(1.162~6.517) 0.021 2.624(1.097~6.277) 0.030 腹水(有vs无) 0.588(0.193~1.796) 0.351 HBsAg(阳性vs阴性) 0.750(0.171~3.282) 0.702 肝外转移(有vs无) 2.715(1.102~6.690) 0.030 0.392(0.157~0.978) 0.045 门静脉癌栓(有vs无) 1.791(0.748~4.289) 0.191 ECOG评分(0 vs 1) 0.228(0.701~4.423) 0.228 Child-Pugh分级(A级vs B级) 0.275(0.326~2.440) 0.275 表 4 OS相关的单因素和多因素分析
Table 4. OS-related univariate and multifactorial analyses
变量 单因素分析 多因素分析 HR(95%CI) P值 HR(95%CI) P值 年龄(≥60岁vs<60岁) 1.138(0.467~2.772) 0.776 AFP(早期无应答vs有应答) 2.639(1.113~6.260) 0.028 2.364(0.957~5.835) 0.062 腹水(有vs无) 0.587(0.191~1.800) 0.351 HBsAg(阳性vs阴性) 0.596(0.133~2.669) 0.498 肝外转移(有vs无) 2.921(1.129~7.235) 0.021 门静脉癌栓(有vs无) 1.941(0.810~4.651) 0.137 2.940(1.152~7.506) 0.024 ECOG评分(1 vs 0) 3.050(1.118~8.318) 0.029 3.000(1.045~8.608) 0.041 Child-Pugh分级(A级vs B级) 0.325(0.053~2.487) 0.279 -
[1] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492. [2] WU T, CHEN L. New advances in the precision diagnosis and treatment of liver cancer[J]. J Clin Hepatol, 2022, 38(3): 497-498. DOI: 10.3969/j.issn.1001-5256.2022.03.001.吴彤, 陈磊. 肝癌精准诊疗新进展[J]. 临床肝胆病杂志, 2022, 38(3): 497-498. DOI: 10.3969/j.issn.1001-5256.2022.03.001. [3] RUMGAY H, ARNOLD M, FERLAY J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040[J]. J Hepatol, 2022, 77(6): 1598-1606. DOI: 10.1016/j.jhep.2022.08.021. [4] European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma[J]. J Hepatol, 2018, 69(1): 182-236. DOI: 10.1016/j.jhep.2018.03.019. [5] WILHELM SM, CARTER C, TANG L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis[J]. Cancer Res, 2004, 64(19): 7099-7109. DOI: 10.1158/0008-5472.CAN-04-1443. [6] GAO R, KALATHUR R, COTO-LLERENA M, et al. YAP/TAZ and ATF4 drive resistance to Sorafenib in hepatocellular carcinoma by preventing ferroptosis[J]. EMBO Mol Med, 2021, 13(12): e14351. DOI: 10.15252/emmm.202114351. [7] ZHOU YY, ZHAO XX, CHEN YY, et al. Tumor immune checkpoint inhibitor and its combination research progress of combination therapy[J]. China pharmacy, 2020, 31(7): 890-896. DOI: 10.6039/j.issn.1001-0408.2020.07.24.周姚邑, 赵新新, 陈沅沅, 等. 肿瘤免疫检查点抑制剂及其联合疗法的研究进展[J]. 中国药房, 2020, 31(7): 890-896. DOI: 10.6039/j.issn.1001-0408.2020.07.24. [8] QIN S, REN Z, MENG Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial[J]. Lancet Oncol, 2020, 21(4): 571-580. DOI: 10.1016/S1470-2045(20)30011-5. [9] KUDO M. Immune checkpoint blockade in hepatocellular carcinoma: 2017 update[J]. Liver Cancer, 2016, 6(1): 1-12. DOI: 10.1159/000449342. [10] ZHAO X, CHEN Q, LIU W, et al. Codelivery of doxorubicin and curcumin with lipid nanoparticles results in improved efficacy of chemotherapy in liver cancer[J]. Int J Nanomedicine, 2014, 10: 257-270. DOI: 10.2147/IJN.S73322. [11] GAO YX, YANG TW, YIN JM, et al. Progress and prospects of biomarkers in primary liver cancer (Review)[J]. Int J Oncol, 2020, 57(1): 54-66. DOI: 10.3892/ijo.2020.5035. [12] HAO X, FAN R, HOU JL. Early warning and accurate screening for the high-risk population of hepatocellular carcinoma[J]. J Clin Hepatol, 2022, 38(3): 499-504. DOI: 10.3969/j.issn.1001-5256.2022.03.002.郝新, 樊蓉, 侯金林. 原发性肝癌高危人群的早期预警和精准筛查[J]. 临床肝胆病杂志, 2022, 38(3): 499-504. DOI: 10.3969/j.issn.1001-5256.2022.03.002. [13] LENCIONI R, LLOVET JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma[J]. Semin Liver Dis, 2010, 30(1): 52-60. DOI: 10.1055/s-0030-1247132. [14] SHIBA S, OKUSAKA T, IKEDA M, et al. Characteristics of 18 patients with hepatocellular carcinoma who obtained a complete response after treatment with sorafenib[J]. Hepatol Res, 2014, 44(13): 1268-1276. DOI: 10.1111/hepr.12297. [15] LIU YC, CHENG TC, BIAN ZL. Progress and challenges of combined immune checkpoint inhibitors in the treatment of hepatocellular carcinoma[J/OL]. Chin J Immunol, 2023. [Online ahead of print]刘一村, 程苕莼, 卞兆连. 联合免疫检查点抑制剂治疗肝细胞癌的进展与挑战[J/OL]. 中国免疫学杂志, 2023. [网络首发] [16] CHANG YS, ADNANE J, TRAIL PA, et al. Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models[J]. Cancer Chemother Pharmacol, 2007, 59(5): 561-574. DOI: 10.1007/s00280-006-0393-4. [17] FU Y, WEI X, LIN L, et al. Adverse reactions of sorafenib, sunitinib, and imatinib in treating digestive system tumors[J]. Thorac Cancer, 2018, 9(5): 542-547. DOI: 10.1111/1759-7714.12608. [18] European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma[J]. J Hepatol, 2012, 56(4): 908-943. DOI: 10.1016/j.jhep.2011.12.001. [19] MOTZER RJ, AGARWAL N, BEARD C, et al. NCCN clinical practice guidelines in oncology: kidney cancer[J]. J Natl Compr Canc Netw, 2009, 7(6): 618-630. DOI: 10.6004/jnccn.2009.0043. [20] FINN RS, IKEDA M, ZHU AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma[J]. J Clin Oncol, 2020, 38(26): 2960-2970. DOI: 10.1200/JCO.20.00808. [21] FINN RS, QIN S, IKEDA M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma[J]. N Engl J Med, 2020, 382(20): 1894-1905. DOI: 10.1056/NEJMoa1915745. [22] KAO WY, CHIOU YY, HUNG HH, et al. Serum alpha-fetoprotein response can predict prognosis in hepatocellular carcinoma patients undergoing radiofrequency ablation therapy[J]. Clin Radiol, 2012, 67(5): 429-436. DOI: 10.1016/j.crad.2011.10.009. [23] SHAO YY, LIN ZZ, HSU C, et al. Early alpha-fetoprotein response predicts treatment efficacy of antiangiogenic systemic therapy in patients with advanced hepatocellular carcinoma[J]. Cancer, 2010, 116(19): 4590-4596. DOI: 10.1002/cncr.25257. [24] SHAO YY, LIU TH, HSU C, et al. Early alpha-foetoprotein response associated with treatment efficacy of immune checkpoint inhibitors for advanced hepatocellular carcinoma[J]. Liver Int, 2019, 39(11): 2184-2189. DOI: 10.1111/liv.14210. [25] PAUL SB, SAHU P, SREENIVAS V, et al. Prognostic role of serial alpha-fetoprotein levels in hepatocellular carcinoma treated with locoregional therapy[J]. Scand J Gastroenterol, 2019, 54(9): 1132-1137. DOI: 10.1080/00365521.2019.1660403. [26] SÁNCHEZ A, ROCES LV, GARCÍA IZ, et al. Value of α-fetoprotein as an early biomarker for treatment response to sorafenib therapy in advanced hepatocellular carcinoma[J]. Oncol Lett, 2018, 15(6): 8863-8870. DOI: 10.3892/ol.2018.8400. [27] PARK JG. Long-term outcomes of patients with advanced hepatocellular carcinoma who achieved complete remission after sorafenib therapy[J]. Clin Mol Hepatol, 2015, 21(3): 287-294. DOI: 10.3350/cmh.2015.21.3.287. [28] LLOVET JM, RICCI S, MAZZAFERRO V, et al. Sorafenib in advanced hepatocellular carcinoma[J]. N Engl J Med, 2008, 359(4): 378-390. DOI: 10.1056/NEJMoa0708857. [29] CHEN LT, LIU TW, CHAO Y, et al. alpha-fetoprotein response predicts survival benefits of thalidomide in advanced hepatocellular carcinoma[J]. Aliment Pharmacol Ther, 2005, 22(3): 217-226. DOI: 10.1111/j.1365-2036.2005.02547.x. -



 PDF下载 ( 2203 KB)
PDF下载 ( 2203 KB)

 下载:
下载:


