| [1] |
|
| [2] |
BUNCHORNTAVAKUL C, MANEERATTANAPORN M, CHAVALITDHAMRONG D. Management of patients with hepatitis C infection and renal disease[J]. World J Hepatol, 2015, 7(2): 213-225.
|
| [3] |
Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for hepatitis C: A 2015 update[J]. J Clin Hepatol, 2015, 31(12): 1961-1979. DOI: 10.3969/j.issn.1001-5256.2015.12.003. |
| [4] |
|
| [5] |
XU JF, ZHANG XH, LIU YL, et al. Influencing factors for hemolytic anemia in patients with chronic hepatitis C treated by ribavirin combined with pegylated interferon-α[J]. J Clin Hepatol, 2019, 35(2): 319-322. DOI: 10.3969/j.issn.1001-5256.2019.02.015. |
| [6] |
|
| [7] |
FELLAY J, THOMPSON AJ, GE D, et al. ITPA gene variants protect against anaemia in patients treated for chronic hepatitis C[J]. Nature, 2010, 464(7287): 405-408. DOI: 10.1038/nature08825. |
| [8] |
OCHI H, MAEKAWA T, ABE H, et al. ITPA polymorphism affects ribavirin-induced anemia and outcomes of therapy-a genome-wide study of Japanese HCV virus patients[J]. Gastroenterology, 2010, 139(4): 1190-1197. DOI: 10.1053/j.gastro.2010.06.071. |
| [9] |
MATSUURA K, TANAKA Y, WATANABE T, et al. ITPA genetic variants influence efficacy of PEG-IFN/RBV therapy in older patients infected with HCV genotype 1 and favourable IL28B type[J]. J Viral Hepat, 2014, 21(7): 466-474. DOI: 10.1111/jvh.12171. |
| [10] |
RAU M, STICKEL F, RUSSMANN S, et al. Impact of genetic SLC28 transporter and ITPA variants on ribavirin serum level, hemoglobin drop and therapeutic response in patients with HCV infection[J]. J Hepatol, 2013, 58(4): 669-675. DOI: 10.1016/j.jhep.2012.11.027. |
| [11] |
CLARK PJ, AGHEMO A, DEGASPERI E, et al. Inosine triphosphatase deficiency helps predict anaemia, anaemia management and response in chronic hepatitis C therapy[J]. J Viral Hepat, 2013, 20(12): 858-866. DOI: 10.1111/jvh.12113. |
| [12] |
MURAKAWA M, ASAHINA Y, NAGATA H, et al. ITPA gene variation and ribavirin-induced anemia in patients with genotype 2 chronic hepatitis C treated with sofosbuvir plus ribavirin[J]. Hepatol Res, 2017, 47(11): 1212-1218. DOI: 10.1111/hepr.12867.URABEA. |
| [13] |
SUZUKI F, SUZUKI Y, AKUTA N, et al. Influence of ITPA polymorphisms on decreases of hemoglobin during treatment with pegylated interferon, ribavirin, and telaprevir[J]. Hepatology, 2011, 53(2): 415-421. DOI: 10.1002/hep.24058. |
| [14] |
MORIO K, IMAMURA M, KAWAKAMI Y, et al. ITPA polymorphism effects on decrease of hemoglobin during sofosbuvir and ribavirin combination treatment for chronic hepatitis C[J]. J Gastroenterol, 2017, 52(6): 746-753. DOI: 10.1007/s00535-016-1279-9. |
| [15] |
URABE A, SAKAMORI R, TAHATA Y, et al. Predictive factors of anemia during sofosbuvir and ribavirin therapy for genotype 2 chronic hepatitis C patients[J]. Hepatol Res, 2019, 49(8): 853-859. DOI: 10.1111/hepr.13354. |



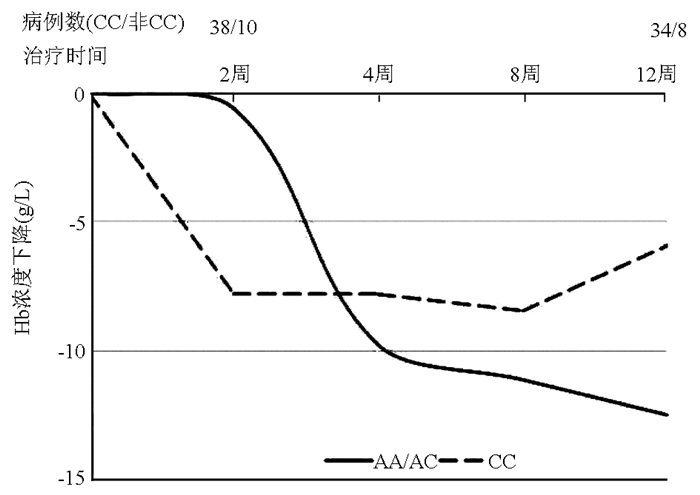




 DownLoad:
DownLoad: