| [1] |
IQBAL U, PERUMPAIL BJ, AKHTAR D, et al. The epidemiology, risk profiling and diagnostic challenges of nonalcoholic fatty liver disease[J]. Medicines (Basel), 2019, 6(1): 41. DOI: 10.3390/medicines6010041. |
| [2] |
SINHA RA, SINGH BK, YEN PM. Direct effects of thyroid hormones on hepatic lipid metabolism[J]. Nat Rev Endocrinol, 2018, 14(5): 259-269. DOI: 10.1038/nrendo.2018.10. |
| [3] |
MANTOVANI A, NASCIMBENI F, LONARDO A, et al. Association between primary hypothyroidism and nonalcoholic fatty liver disease: A systematic review and meta-analysis[J]. Thyroid, 2018, 28(10): 1270-1284. DOI: 10.1089/thy.2018.0257. |
| [4] |
XU R, HUANG F, ZHANG S, et al. Thyroid function, body mass index, and metabolic risk markers in euthyroid adults: A cohort study[J]. BMC Endocr Disord, 2019, 19(1): 58. DOI: 10.1186/s12902-019-0383-2. |
| [5] |
RAHBAR AR, KALANTARHORMOZI M, IZADI F, et al. Relationship between Body Mass Index, Waist-to-Hip Ratio, and Serum Lipid Concentrations and Thyroid-Stimulating Hormone in the euthyroid adult population[J]. Iran J Med Sci, 2017, 42(3): 301-305.
|
| [6] |
National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association; Fatty Liver Expert Committee, Chinese Medical Doctor Association. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: A 2018 update[J]. J Clin Hepatol, 2018, 34(5): 947-957. DOI: 10.3969/j.issn.1001-5256.2018.05.007. |
| [7] |
YOUNOSSI ZM, KOENIG AB, ABDELATIF D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes[J]. Hepatology, 2016, 64(1): 73-84. DOI: 10.1002/hep.28431. |
| [8] |
WANG YH, GAO Y. Research progress in diagnosis and treatment of non-alcoholic fatty liver disease combinated with type 2 diabetes mellitus[J]. J Jilin Univ(Med Edit), 2020, 46(6): 1324-1331. DOI: 10.13481/j.1671-587x.20200634. |
| [9] |
DUNTAS LH, BRENTA G. A Renewed focus on the association between thyroid hormones and lipid metabolism[J]. Front Endocrinol (Lausanne), 2018, 9: 511. DOI: 10.3389/fendo.2018.00511. |
| [10] |
SINHA RA, SINGH BK, YEN PM. Reciprocal crosstalk between autophagic and endocrine signaling in metabolic homeostasis[J]. Endocr Rev, 2017, 38(1): 69-102. DOI: 10.1210/er.2016-1103. |
| [11] |
CHI HC, TSAI CY, TSAI MM, et al. Molecular functions and clinical impact of thyroid hormone-triggered autophagy in liver-related diseases[J]. J Biomed Sci, 2019, 26(1): 24. DOI: 10.1186/s12929-019-0517-x. |
| [12] |
CHAKRAVARTHY MV, NEUSCHWANDER-TETRI BA. The metabolic basis of nonalcoholic steatohepatitis[J]. Endocrinol Diabetes Metab, 2020, 3(4): e00112. DOI: 10.1002/edm2.112. |
| [13] |
FERRANDINO G, KASPARI RR, SPADARO O, et al. Pathogenesis of hypothyroidism-induced NAFLD is driven by intra- and extrahepatic mechanisms[J]. Proc Natl Acad Sci U S A, 2017, 114(43): e9172-e9180. DOI: 10.1073/pnas.1707797114. |
| [14] |
VERGANI L. Lipid lowering effects of iodothyronines: In vivo and in vitro studies on rat liver[J]. World J Hepatol, 2014, 6(4): 169-177. DOI: 10.4254/wjh.v6.i4.169. |
| [15] |
LIU Y, WANG W, YU X, et al. Thyroid function and risk of non-alcoholic fatty liver disease in euthyroid subjects[J]. Ann Hepatol, 2018, 17(5): 779-788. DOI: 10.5604/01.3001.0012.3136. |
| [16] |
BORGES-CANHA M, NEVES JS, MENDONÇA F, et al. Thyroid function and the risk of non-alcoholic fatty liver disease in morbid obesity[J]. Front Endocrinol (Lausanne), 2020, 11: 572128. DOI: 10.3389/fendo.2020.572128. |
| [17] |
van den BERG EH, van TIENHOVEN-WIND LJ, AMINI M, et al. Higher free triiodothyronine is associated with non-alcoholic fatty liver disease in euthyroid subjects: The Lifelines Cohort Study[J]. Metabolism, 2017, 67: 62-71. DOI: 10.1016/j.metabol.2016.11.002. |
| [18] |
JARUVONGVANICH V, SANGUANKEO A, UPALA S. Nonalcoholic fatty liver disease is not associated with thyroid hormone levels and hypothyroidism: A systematic review and meta-analysis[J]. Eur Thyroid J, 2017, 6(4): 208-215. DOI: 10.1159/000454920. |
| [19] |
DUNTAS LH. Thyroid function in aging: A discerning approach[J]. Rejuvenation Res, 2018, 21(1): 22-28. DOI: 10.1089/rej.2017.1991. |
| [20] |
LIU G, LIANG L, BRAY GA, et al. Thyroid hormones and changes in body weight and metabolic parameters in response to weight loss diets: The POUNDS LOST trial[J]. Int J Obes (Lond), 2017, 41(6): 878-886. DOI: 10.1038/ijo.2017.28. |
| [21] |
BRIL F, KADIYALA S, PORTILLO SANCHEZ P, et al. Plasma thyroid hormone concentration is associated with hepatic triglyceride content in patients with type 2 diabetes[J]. J Investig Med, 2016, 64(1): 63-68. DOI: 10.1136/jim-2015-000019. |
| [22] |
ITTERMANN T, HARING R, WALLASCHOFSKI H, et al. Inverse association between serum free thyroxine levels and hepatic steatosis: Results from the Study of Health in Pomerania[J]. Thyroid, 2012, 22(6): 568-574. DOI: 10.1089/thy.2011.0279. |
| [23] |
MARSILI A, AGUAYO-MAZZUCATO C, CHEN T, et al. Mice with a targeted deletion of the type 2 deiodinase are insulin resistant and susceptible to diet induced obesity[J]. PLoS One, 2011, 6(6): e20832. DOI: 10.1371/journal.pone.0020832. |
| [24] |
FERNANDES GW, BOCCO B, FONSECA TL, et al. The foxo1-inducible transcriptional repressor Zfp125 causes hepatic steatosis and hypercholesterolemia[J]. Cell Rep, 2018, 22(2): 523-534. DOI: 10.1016/j.celrep.2017.12.053. |
| [25] |
SAPONARO F, SESTITO S, RUNFOLA M, et al. Selective thyroid hormone receptor-beta (TRβ) agonists: New perspectives for the treatment of metabolic and neurodegenerative disorders[J]. Front Med (Lausanne), 2020, 7: 331. DOI: 10.3389/fmed.2020.00331. |



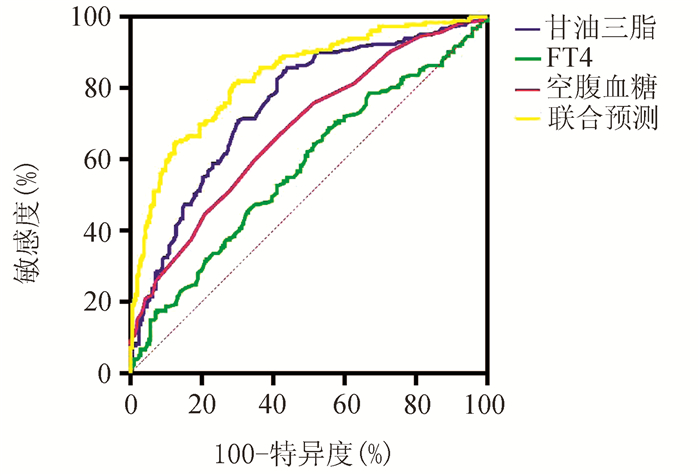




 DownLoad:
DownLoad: