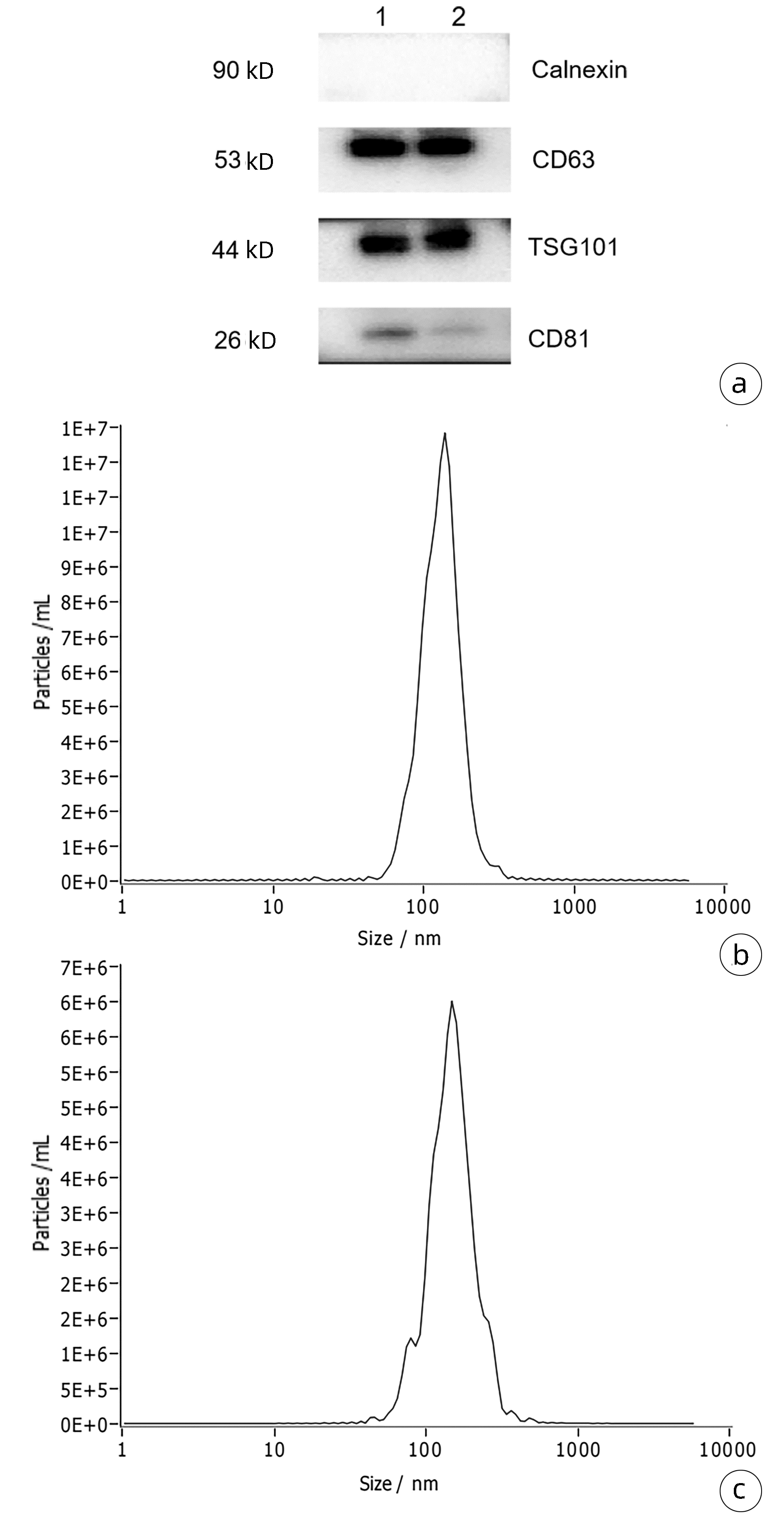
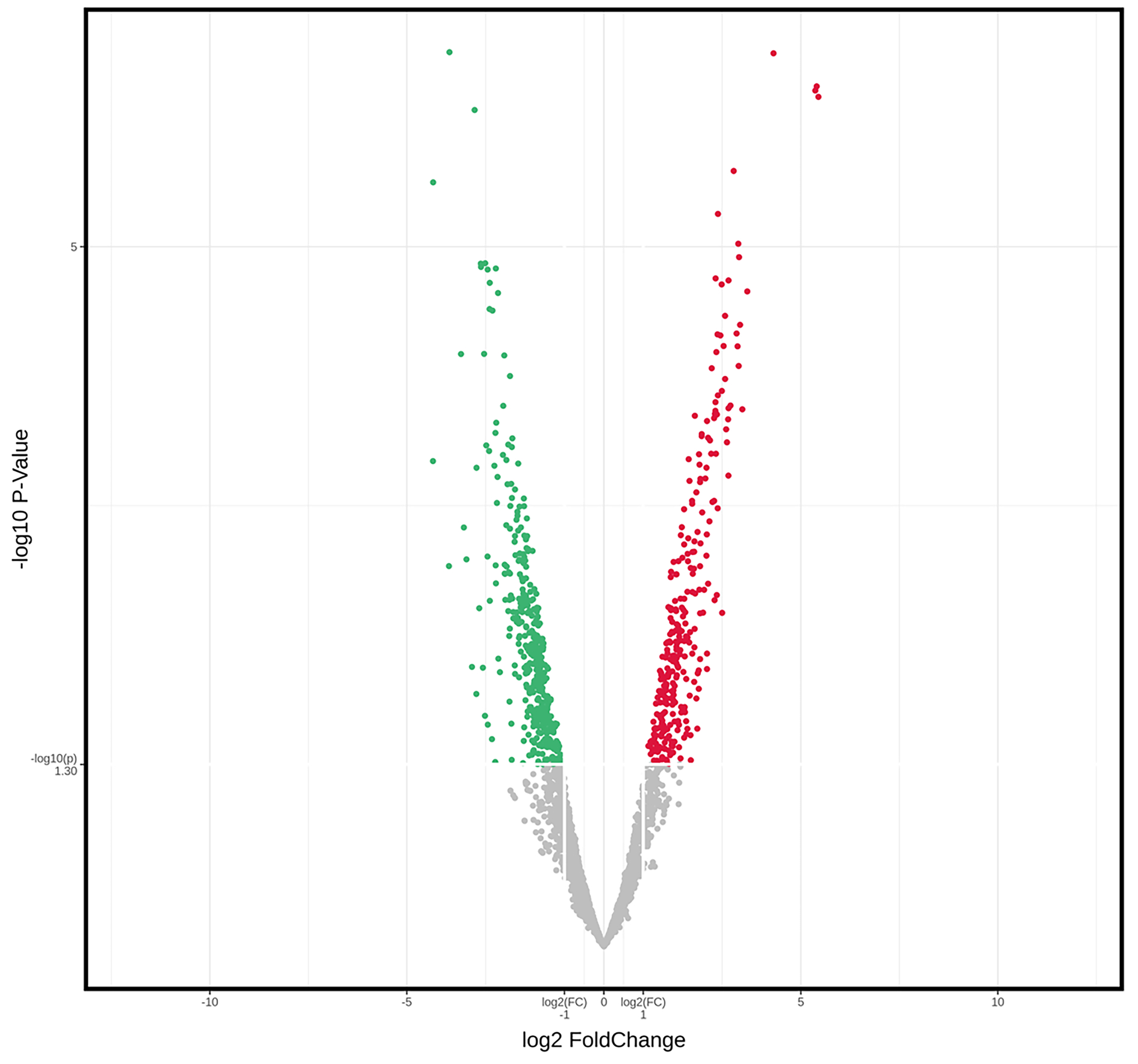
慢性乙型肝炎与HBV相关慢加急性肝衰竭患者血浆外泌体差异miRNA的生物信息学分析
DOI: 10.3969/j.issn.1001-5256.2023.08.013
Differentially expressed microRNAs in plasma exosomes from patients with chronic hepatitis B or hepatitis B virus-related acute-on-chronic liver failure: A bioinformatics analysis
-
摘要:
目的 探讨慢性乙型肝炎(CHB)患者与HBV相关慢加急性肝衰竭(HBV-ACLF)患者血浆外泌体microRNA(miRNA)表达谱差异,分析其功能及生物学过程,以期获得可用于HBV-ACLF临床诊断的参考依据。 方法 选取2021年10月—2022年6月青岛市市立医院感染科住院的6例CHB患者及青岛市第六人民医院血液净化中心接受治疗的6例HBV-ACLF患者。运用Illumina高通量测序技术对这些患者的血浆外泌体miRNA进行检测,筛选差异miRNA并进行功能富集分析,分析其参与的生物学过程。检测得到的外泌体差异miRNA以倍数上调>2倍或下调>2倍且P<0.05为标准筛选。计量资料两组间比较采用Mann-Whitney U秩和检验。计数资料两组间比较采用χ2检验。 结果 筛选差异miRNA共249种,与CHB组相比,HBV-ACLF组上调miRNA 126种,下调miRNA 123种。生物信息学分析结果显示,这些差异表达miRNA主要参与了性腺发育、调控蛋白质稳定性、细胞对外界刺激反应等生物学过程,并与乙型肝炎、蛋白多糖在癌症中的作用、调节干细胞多能性、MAPK、Hippo、TNF、脂质代谢等信号通路密切相关。 结论 通过Illumina高通量测序技术筛选出的差异miRNA可能作为HBV-ACLF早期诊断及预后判断的生物标志物。 Abstract:Objective To investigate the differences in plasma exosomal microRNA (miRNA) expression profile between patients with chronic hepatitis B (CHB) and those with hepatitis B virus-related acute-on-chronic liver failure (HBV-ACLF), as well as their functions and biological processes, and to provide a reference for the clinical diagnosis of HBV-ACLF. Methods Six patients with CHB who were hospitalized in Department of Infectious Diseases, Qingdao Municipal Hospital, and six patients with HBV-ACLF who were treated in Blood Purification Center of Qingdao Sixth People's Hospital from October 2021 to June 2022 were enrolled. Illumina high-throughput sequencing was used to analyze the plasma exosomal miRNAs of these patients to obtain the differentially expressed miRNAs between the two groups. The differentially expressed miRNAs were screened, and a functional enrichment analysis was performed to identify the biological processes involving such miRNAs. The Mann-Whitney U rank sum test was used for comparison of continuous data between two groups, and the chi-square test was used for comparison of categorical data between groups. Results A total of 249 differentially expressed exosomal miRNAs were obtained according to the criteria of upregulation >2-fold or downregulation >2-fold, with P < 0.05, and compared with the CHB group, there were 126 upregulated miRNAs and 123 downregulated miRNAs. The bioinformatics analysis showed that these differentially expressed miRNAs were mainly involved in the biological processes such as gonadal development, regulation of protein stability, and cellular response to external stimuli, and they were closely associated with the signaling pathways such as hepatitis B, the role of proteoglycans in cancer, regulation of stem cell pluripotency, MAPK, Hippo, TNF, and lipid metabolism. Conclusion The differentially expressed miRNAs identified by Illumina high-throughput sequencing may be used as biomarkers for the early diagnosis and prognostic evaluation of HBV-ACLF. -
Key words:
- Hepatitis B, Chronic /
- Acute-On-Chronic Liver Failure /
- Exosomes /
- MicroRNAs
-
表 1 两组患者一般资料比较
Table 1. Comparison of baseline clinical characteristics between CHB group and HBV-ACLF group
项目 CHB组(n=6) HBV-ACLF组(n=6) 统计值 P值 男性[例(%)] 4(66.67) 4(66.67) >0.05 年龄(岁) 56.00(48.75~65.25) 55.50(50.50~66.00) Z=-0.241 0.810 ALT(U/L) 23.94(15.47~36.38) 487.00(197.25~866.25) Z=-2.882 0.004 AST(U/L) 23.09(19.63~32.06) 249.00(194.75~692.00) Z=-2.882 0.004 TBil(μmol/L) 13.05(10.28~18.63) 284.90(268.08~355.93) Z=-2.882 0.004 DBil(μmol/L) 2.92(2.53~3.64) 209.20(184.03~226.58) Z=-2.882 0.004 WBC(×109/L) 4.92(4.09~5.62) 5.46(4.35~8.08) Z=-0.801 0.423 RBC(×1012/L) 4.56(4.30~4.73) 4.17(3.72~4.85) Z=-0.642 0.521 PLT(×109/L) 164.00(144.75~202.50) 86.00(50.25~148.75) Z=-2.082 0.037 PT(s) 12.50(11.53~13.55) 23.25(17.90~38.30) Z=-2.882 0.004 APTT(s) 31.15(28.63~39.25) 42.35(35.03~53.08) Z=-2.082 0.037 注:APTT,活化部分凝血活酶时间。 表 2 HBV-ACLF患者差异表达的miRNA
Table 2. Differentially expressed miRNAs in HBV-ACLF patients
上调miRNA 差异倍数 P值 下调miRNA 差异倍数 P值 hsa-miR-6886-3p 43.586 0.000 00 hsa-miR-3689b-5p 0.119 0.010 19 hsa-miR-6880-3p 42.256 0.000 00 hsa-miR-1298-5p 0.115 0.000 01 hsa-miR-10398-5p 41.267 0.000 00 hsa-miR-4754 0.115 0.000 01 hsa-miR-122b-3p 19.775 0.000 00 hsa-miR-183-3p 0.112 0.003 83 hsa-miR-411-3p 12.467 0.000 02 hsa-miR-449b-5p 0.106 0.000 38 hsa-miR-4327 11.427 0.000 15 hsa-miR-4317 0.106 0.015 70 hsa-miR-7111-3p 10.981 0.000 04 hsa-miR-5689 0.103 0.000 00 hsa-let-7b-3p 10.785 0.000 01 hsa-miR-520f-5p 0.098 0.010 06 hsa-miR-335-3p 10.718 0.000 07 hsa-miR-4291 0.089 0.001 71 hsa-miR-4646-5p 10.647 0.000 01 hsa-miR-96-3p 0.085 0.001 01 hsa-miR-6887-5p 10.511 0.000 05 hsa-miR-631 0.081 0.000 06 hsa-miR-1281 10.327 0.000 04 hsa-miR-3678-3p 0.066 0.000 00 hsa-miR-4510 9.819 0.000 00 hsa-miR-6755-5p 0.066 0.001 92 hsa-miR-2114-3p 9.280 0.000 14 hsa-miR-6841-5p 0.050 0.000 00 hsa-miR-6760-5p 8.961 0.000 02 hsa-miR-4795-5p 0.049 0.000 34 -
[1] TANG Y, LIANG H, ZENG G, et al. Advances in new antivirals for chronic hepatitis B[J]. Chin Med J (Engl), 2022, 135(5): 571-583. DOI: 10.1097/CM9.0000000000001994. [2] ARROYO V, MOREAU R, JALAN R. Acute-on-chronic liver failure[J]. N Engl J Med, 2020, 382(22): 2137-2145. DOI: 10.1056/NEJMra1914900. [3] CONSOLE L, SCALISE M, INDIVERI C. Exosomes in inflammation and role as biomarkers[J]. Clin Chim Acta, 2019, 488: 165-171. DOI: 10.1016/j.cca.2018.11.009. [4] Chinese Society of Infectious Diseases, Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007.中华医学会感染病学分会, 中华医学会肝病学分会. 慢性乙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. [5] Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Guideline for diagnosis and treatment of liver failure(2018)[J]. J Clin Hepatol, 2019, 35(1): 38-44. DOI: 10.3969/j.issn.1001-5256.2019.01.007.中华医学会感染病学分会肝衰竭与人工肝学组, 中华医学会肝病学分会重型肝病与人工肝学组. 肝衰竭诊治指南(2018年版)[J]. 临床肝胆病杂志, 2019, 35(1): 38-44. DOI: 10.3969/j.issn.1001-5256.2019.01.007. [6] GURUNATHAN S, KANG MH, JEYARAJ M, et al. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes[J]. Cells, 2019, 8(4): 307. DOI: 10.3390/cells8040307. [7] MORI MA, LUDWIG RG, GARCIA-MARTIN R, et al. Extracellular miRNAs: from biomarkers to mediators of physiology and disease[J]. Cell Metab, 2019, 30(4): 656-673. DOI: 10.1016/j.cmet.2019.07.011. [8] ZHANG J, LI S, LI L, et al. Exosome and exosomal microRNA: trafficking, sorting, and function[J]. Genomics Proteomics Bioinformatics, 2015, 13(1): 17-24. DOI: 10.1016/j.gpb.2015.02.001. [9] CHEN W, YAN ZH, WANG YM, et al. Genome-wide microarray-based analysis of miRNAs expression in patients with acute-on-chronic liver failure[J]. Hepatobiliary Pancreat Dis Int, 2014, 13(1): 32-39. DOI: 10.1016/s1499-3872(14)60004-7. [10] HU J, XU Y, HAO J, et al. MiR-122 in hepatic function and liver diseases[J]. Protein Cell, 2012, 3(5): 364-371. DOI: 10.1007/s13238-012-2036-3. [11] TANIMIZU N, KOBAYASHI S, ICHINOHE N, et al. Downregulation of miR122 by grainyhead-like 2 restricts the hepatocytic differentiation potential of adult liver progenitor cells[J]. Development, 2014, 141(23): 4448-4456. DOI: 10.1242/dev.113654. [12] TU WL, YOU LR, TSOU AP, et al. Pten haplodeficiency accelerates liver tumor growth in miR-122a-Null mice via expansion of periportal hepatocyte-like cells[J]. Am J Pathol, 2018, 188(11): 2688-2702. DOI: 10.1016/j.ajpath.2018.07.019. [13] KRAUSKOPF J, CAIMENT F, CLAESSEN SM, et al. Application of high-throughput sequencing to circulating microRNAs reveals novel biomarkers for drug-induced liver injury[J]. Toxicol Sci, 2015, 143(2): 268-276. DOI: 10.1093/toxsci/kfu232. [14] BAI S, NASSER MW, WANG B, et al. MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib[J]. J Biol Chem, 2009, 284(46): 32015-32027. DOI: 10.1074/jbc.M109.016774. [15] JOPLING CL, YI M, LANCASTER AM, et al. Modulation of hepatitis C virus RNA abundance by a liver-specific microRNA[J]. Science, 2005, 309(5740): 1577-1581. DOI: 10.1126/science.1113329. [16] CHEN S, YANG L, PAN A, et al. Inhibitory effect on the hepatitis B cells through the regulation of miR-122-MAP3K2 signal pathway[J]. An Acad Bras Cienc, 2019, 91(2): e20180941. DOI: 10.1590/0001-3765201920180941. [17] WEN Y, PENG SF, FU L, et al. Serum levels of miRNA in patients with hepatitis B virus-associated acute-on-chronic liver failure[J]. Hepatobiliary Pancreat Dis Int, 2018, 17(2): 126-132. DOI: 10.1016/j.hbpd.2018.03.004. [18] WU L, NGUYEN LH, ZHOU K, et al. Precise let-7 expression levels balance organ regeneration against tumor suppression[J]. Elife, 2015, 4: e09431. DOI: 10.7554/eLife.09431. [19] MCDANIEL K, HUANG L, SATO K, et al. The let-7/Lin28 axis regulates activation of hepatic stellate cells in alcoholic liver injury[J]. J Biol Chem, 2017, 292(27): 11336-11347. DOI: 10.1074/jbc.M116.773291. [20] MATSUURA K, AIZAWA N, ENOMOTO H, et al. Circulating let-7 levels in serum correlate with the severity of hepatic fibrosis in chronic hepatitis C[J]. Open Forum Infect Dis, 2018, 5(11): ofy268. DOI: 10.1093/ofid/ofy268. [21] SANDHU GK, MCMILLIN M, FRAMPTON G, et al. Let-7f-dependent suppression of neuronal IGF1 by aberrant TGFβ1 signaling contributes to the neurological decline observed during acute liver failure[J]. J Gastroenterology, 2017, 152(5): S1066. DOI: 10.1016/s0016-5085(17)33601-6. [22] YANG S, CHEN Z, FAN D, et al. Retracted Article: MiR-182-5p and miR-96-5p increased hepatocellular carcinoma cell mobility, proliferation and cisplatin resistance partially by targeting RND3[J]. RSC Adv, 2018, 8(61): 34973-34983. DOI: 10.1039/c8ra07055e. [23] CHANDEL R, DAS A, CHAWLA YK, et al. Mo1477 Progression of hepatocellular carcinoma is associated with the up regulation of rno-miR-96/182/183 cluster in liver of wistar rats[J]. J Gastroenterology, 2016, 150(4): S1125. DOI: 10.1016/s0016-5085(16)33799-4 [24] MANDAL R, HARDIN H, BAUS R, et al. Analysis of miR-96 and miR-133a expression in gastrointestinal neuroendocrine neoplasms[J]. Endocr Pathol, 2017, 28(4): 345-350. DOI: 10.1007/s12022-017-9504-5. [25] SHI Y, JIA M, XU L, et al. miR-96 and autophagy are involved in the beneficial effect of grape seed proanthocyanidins against high-fat-diet-induced dyslipidemia in mice[J]. Phytother Res, 2019, 33(4): 1222-1232. DOI: 10.1002/ptr.6318. [26] BAJAJ JS, REDDY KR, O'LEARY JG, et al. Serum levels of metabolites produced by intestinal microbes and lipid moieties independently associated with acute-on-chronic liver failure and death in patients with cirrhosis[J]. Gastroenterology, 2020, 159(5): 1715-1730. e12. DOI: 10.1053/j.gastro.2020.07.019. -
 慢性乙型肝炎与HBV相关慢加急性肝衰竭患者血浆外泌体差异miRNA的生物信息学分析.pdf
慢性乙型肝炎与HBV相关慢加急性肝衰竭患者血浆外泌体差异miRNA的生物信息学分析.pdf

-



 PDF下载 ( 5016 KB)
PDF下载 ( 5016 KB)


 下载:
下载: