鞘氨醇激酶1(Sphk1)抑制剂在肝纤维化大鼠模型中的作用机制
DOI: 10.3969/j.issn.1001-5256.2023.08.018
Mechanism of action of sphingosine kinase 1 inhibitor in a rat model of liver fibrosis
-
摘要:
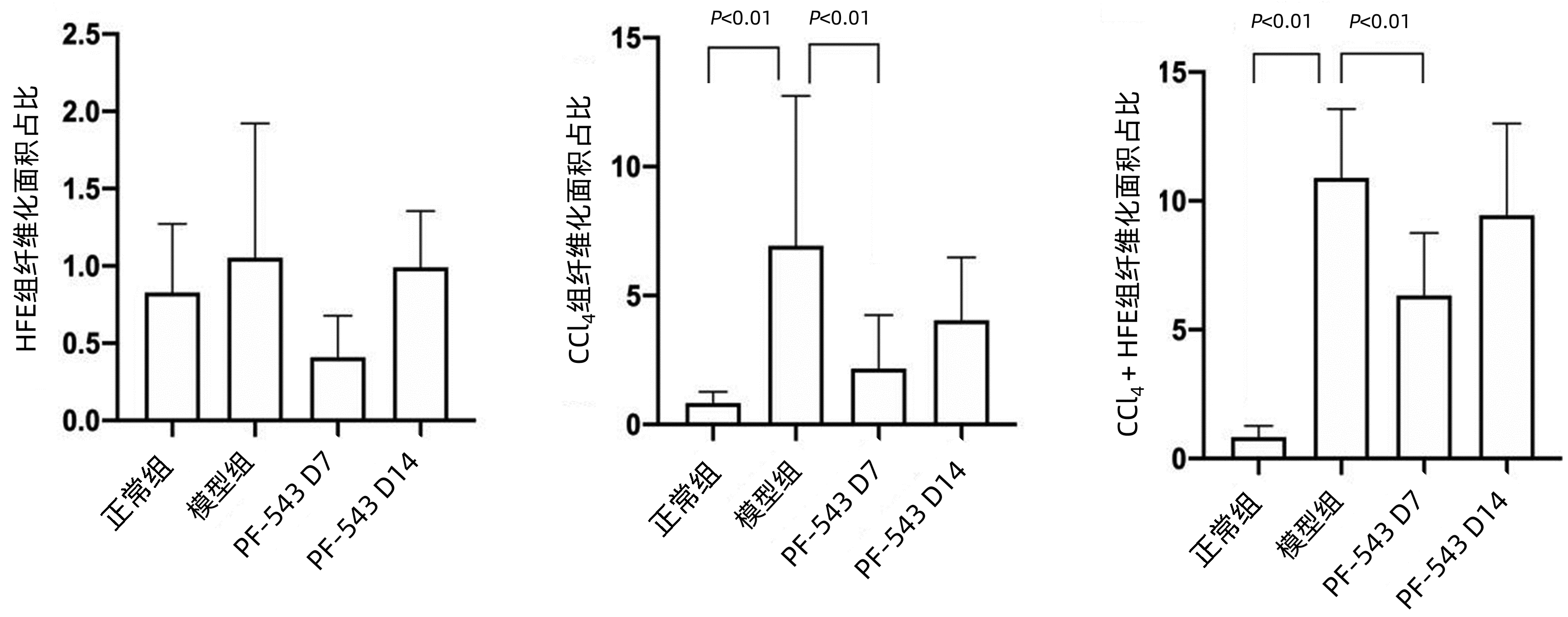
目的 探讨鞘氨醇激酶1(SphK1)抑制剂在不同肝纤维化大鼠模型中的治疗作用。 方法 170只SD大鼠随机分为4组:正常组(30只)、HFE组(给予高脂乳剂,45只)、CCl4组(CCl4诱导,45只)、CCl4+HFE组(HFE联合CCl4,50只),经病理及实验室检查证实造模成功后,分别给予SphK1抑制剂PF-543,同组别以生理盐水作为对照,分别在第1、7、14天取肝组织进行Masson染色,比较肝纤维化面积占比、透射电镜下观察自噬小体形成情况、Real-time PCR检测肝纤维化及自噬相关标志物mRNA表达水平。计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD检验。相关性分析采用Spearman相关分析。 结果 与正常组(0.57±0.13)相比,CCl4组(6.93±5.81)和HFE+CCl4组(10.89±2.67)肝纤维化面积占比均升高(P值均<0.01)。PF-543干预第7天可显著降低CCl4组及HFE+CCl4组纤维化病变面积(P值均<0.01)。与正常组相比,HFE组、CCl4组和HFE+CCl4组的SphK1表达水平均明显降低(P值均<0.01),HFE+CCl4组的α平滑肌肌动蛋白、转化生长因子-β、α1-Ⅰ型胶原mRNA表达水平均明显升高(P值均<0.01),干预后各指标水平无明显变化(P值均>0.05)。Atg5、Atg12、Becn1的表达水平与肝纤维化病变面积均呈负相关(r分别为-0.715、-0.640、-0.632,P值均<0.01);SphK1的表达水平与Atg5、Atg12、Becn1、Map1lc3a的mRNA表达均呈正相关(r分别为0.603、0.561、0.510、0.498,P值均<0.01)。电镜示:CCl4组肝组织轻微水肿,细胞器丰富,轻度肿胀,以粗面内质网扩张为主。该视野可见9个自噬溶酶体结构。在PF-543干预第7天后,未见典型自噬结构。HFE+CCl4组肝组织轻度水肿,结构不清晰,粗面内质网数量丰富,明显扩张,表面附着核糖体颗粒较少。该视野可见6个自噬溶酶体结构。在PF-543干预第7天后,该视野未见典型自噬结构。 结论 PF-543对自噬有显著抑制作用,并同时伴有纤维化面积的减少。提示靶向SphK1,可影响到肝脏的自噬水平,并进而缓解两种肝纤维化模型的纤维化状态。 Abstract:Objective To investigate the therapeutic effect of sphingosine kinase 1 (SphK1) inhibitor in different liver fibrosis models. Methods A total of 170 Sprague-Dawley rats were randomly divided into normal group (30 rats), HFE group (45 rats given high-fat emulsion), CCl4 group (45 rats induced by CCl4), and CCl4+HFE group (50 rats given HFE and CCl4). After successful modeling confirmed by pathology and laboratory examination, the SphK1 inhibitor PF-543 was given, and the rats in the same group were given normal saline as control. Liver tissue samples were collected on days 1, 7, and 14 for Masson staining; the percentage of liver fibrosis area was compared; the formation of autophagosome was observed under a transmission electron microscope; real-time PCR was used to measure the mRNA expression levels of markers associated with liver fibrosis and autophagy. A one-way analysis of variance was used for comparison between multiple groups, and the LSD method was used for further comparison between two groups; a Spearman correlation analysis was also performed. Results Compared with the normal group, the CCl4 group and the HFE+CCl4 group had a significant increase in the percentage of liver fibrosis area (6.93±5.81/10.89±2.67 vs 0.57±0.13, both P < 0.01), the CCl4 group and the HFE+CCl4 group had a significant reduction in the percentage of liver fibrosis area on day 7 (both P < 0.01). Compared with the normal group, the HFE group, the CCl4 group, and the HFE+CCl4 group had a significant reduction in the expression level of SphK1 (all P < 0.01), and the HFE+CCl4 group had significant increases in the mRNA expression levels of alpha-smooth muscle actin, transforming growth factor-β, and collagen type Ⅰ alpha 1 (all P < 0.01), while there were no significant changes in these indices after intervention (all P > 0.05). The expression levels of Atg5, Atg12, and Becn1 were negatively correlated with the area of liver fibrosis (r=-0.715, -0.640, and -0.632, all P < 0.01), and the expression level of SphK1 was positively correlated with the mRNA expression of Atg5, Atg12, Becn1, and Map1lc3a (r=0.603, 0.561, 0.510, and 0.498, all P < 0.01). Electron microscopy showed that the CCL4 group had slight edema, abundant organelles, and mild swelling in liver tissue, mainly the expansion of rough endoplasmic reticulum, and nine autolysosome (ASS) structures were seen in this field, while no typical ASS structure was observed on day 7 of PF-543 intervention. The HFE+CCL4 group had mild edema, unclear structure, and abundant rough endoplasmic reticulum with marked expansion and few ribosome particles attached to its surface in liver tissue, and 6 ASS structures were seen in this field, while no typical ASS structure was observed on day 7 of PF-543 intervention. Conclusion PF-543 significantly inhibits autophagy and is associated with a reduction in fibrosis area. It is suggested that targeting SphK1 can affect the level of liver autophagy, thereby alleviating the state of liver fibrosis in two liver fibrosis models. -
Key words:
- Hepatic Fibrosis /
- Sphingosine Kinase 1 /
- Autophagy /
- Models, Animal
-
表 1 各基因PCR引物序列
Table 1. PCR primer sequence of each gene
引物名称 引物序列 产物长度
(bp)Acta2-F 5′-GGCTGTGATCTCCTTCTG-3′ 139 Acta2-R 5′-CATCCACGAAACCACCTAT-3′ 139 Atg5-F 5′-ACAACTGAACGGCCTTTC-3′ 103 Atg5-R 5′-CTGCGGAAGGACAGACTT-3′ 103 Atg12-F 5′-CATTCTACATCCCAAACACATC-3′ 116 Atg12-R 5′-AGTTATTGGATTGGCACTGT-3′ 116 Becn1-F 5′-TTTCCACCTCTTCTTTGAAC-3′ 111 Becn1-R 5′-AGTTGCCGTTGTACTGTT-3′ 111 Col1a1-F 5′-CTAACCAAGGCTGCAACC-3′ 113 Col1a1-R 5′-GCTGATGTACCAGTTCTTCT-3′ 113 Fn1-F 5′-TGAAATGACCACTGCCAAA-3′ 137 Fn1-R 5′-GAACCAGCCTACGGATGA-3′ 137 Map1lc3a -F 5′-TAAGGAGGTGCAGCAGAT-3′ 153 Map1lc3a -R 5′-CGGATGATCTTGACCAACT-3′ 153 SphK1-F 5′-CAACACCGATAAGCAGCT-3′ 109 SphK1-R 5′-TGGTTGCATAGCCAGGTC-3′ 109 TGF-β1-F 5′-CAGAGAAGAACTGCTGTGTA-3′ 134 TGF-β1-R 5′-GTCCAGGCTCCAAATGTAG-3′ 134 Rat-GAPDH-F 5′-TCTCTTGTGACAAAGTGGACA-3′ 128 Rat-GAPDH-R 5′-CCCATTCTCAGCCTTGACTGT-3′ 128 注:Col1a1,α1-Ⅰ型胶原;Fn1,纤维连接蛋白;TGF-β,转化生长因子-β。 -
[1] DEVARAJ E, PERUMAL E, SUBRAMANIYAN R, et al. Liver fibrosis: Extracellular vesicles mediated intercellular communication in perisinusoidal space[J]. Hepatology, 2022, 76(1): 275-285. DOI: 10.1002/hep.32239. [2] XUE R, FAN JG. Brief introduction of an international expert consensus statement: A new definition of metabolic associated fatty liver disease[J]. J Clin Hepatol, 2020, 36(6): 1224-1227. DOI: 10.3969/j.issn.1001-5256.2020.06.007.薛芮, 范建高. 代谢相关脂肪性肝病新定义的国际专家共识简介[J]. 临床肝胆病杂志, 2020, 36(6): 1224-1227. DOI: 10.3969/j.issn.1001-5256.2020.06.007. [3] ESCUDERO-CASAO M, CARDONA A, BELTRAN-DEBON R, et al. Fluorinated triazole-containing sphingosine analogues. Syntheses and in vitro evaluation as SPHK inhibitors[J]. Org Biomol Chem, 2018, 16(39): 7230-7235. DOI: 10.1039/c8ob01867g. [4] ANDERSON AK, LAMBERT JM, MONTEFUSCO DJ, et al. Depletion of adipocyte sphingosine kinase 1 leads to cell hypertrophy, impaired lipolysis, and nonalcoholic fatty liver disease[J]. J Lipid Res, 2020, 61(10): 1328-1340. DOI: 10.1194/jlr.RA120000875. [5] AVNI D, HARIKUMAR KB, SANYAL AJ, et al. Deletion or inhibition of SphK1 mitigates fulminant hepatic failure by suppressing TNFα-dependent inflammation and apoptosis[J]. FASEB J, 2021, 35(3): e21415. DOI: 10.1096/fj.202002540R. [6] LI L, WANG H, ZHANG J, et al. SPHK1 deficiency protects mice from acetaminophen-induced ER stress and mitochondrial permeability transition[J]. Cell Death Differ, 2020, 27(6): 1924-1937. DOI: 10.1038/s41418-019-0471-x. [7] GENG T, SUTTER A, HARLAND MD, et al. SphK1 mediates hepatic inflammation in a mouse model of NASH induced by high saturated fat feeding and initiates proinflammatory signaling in hepatocytes[J]. J Lipid Res, 2015, 56(12): 2359-2371. DOI: 10.1194/jlr.M063511. [8] LI Q, QIAN J, LI Y, et al. Generation of sphingosine-1-phosphate by sphingosine kinase 1 protects nonalcoholic fatty liver from ischemia/reperfusion injury through alleviating reactive oxygen species production in hepatocytes[J]. Free Radic Biol Med, 2020, 159: 136-149. DOI: 10.1016/j.freeradbiomed.2020.07.004. [9] CHEN Y, AZAD MB, GIBSON SB. Methods for detecting autophagy and determining autophagy-induced cell death[J]. Can J Physiol Pharmacol, 2010, 88(3): 285-295. DOI: 10.1139/Y10-010. [10] LAVIEU G, SCARLATTI F, SALA G, et al. Regulation of autophagy by sphingosine kinase 1 and its role in cell survival during nutrient starvation[J]. J Biol Chem, 2006, 281(13): 8518-8527. DOI: 10.1074/jbc.M506182200. [11] HUANG LS, NATARAJAN V. Sphingolipids in pulmonary fibrosis[J]. Adv Biol Regul, 2015, 57: 55-63. DOI: 10.1016/j.jbior.2014.09.008. [12] CHERESH P, KIM SJ, HUANG LS, et al. The sphingosine kinase 1 inhibitor, PF543, mitigates pulmonary fibrosis by reducing lung epithelial cell mtDNA damage and recruitment of fibrogenic monocytes[J]. Int J Mol Sci, 2020, 21(16): 5595. DOI: 10.3390/ijms21165595. [13] HA AW, SUDHADEVI T, EBENEZER DL, et al. Neonatal therapy with PF543, a sphingosine kinase 1 inhibitor, ameliorates hyperoxia-induced airway remodeling in a murine model of bronchopulmonary dysplasia[J]. Am J Physiol Lung Cell Mol Physiol, 2020, 319(3): L497-L512. DOI: 10.1152/ajplung.00169.2020. [14] IMBERT C, MONTFORT A, FRAISSE M, et al. Resistance of melanoma to immune checkpoint inhibitors is overcome by targeting the sphingosine kinase-1[J]. Nat Commun, 2020, 11(1): 437. DOI: 10.1038/s41467-019-14218-7. [15] SUKOCHEVA OA, FURUYA H, NG ML, et al. Sphingosine kinase and sphingosine-1-phosphate receptor signaling pathway in inflammatory gastrointestinal disease and cancers: A novel therapeutic target[J]. Pharmacol Ther, 2020, 207: 107464. DOI: 10.1016/j.pharmthera.2019.107464. [16] JIN LM, HUANG DS, LIU YX, et al. The effects of sphingosine kinase/1-phosphate sphingosine signaling on hepatic ischemia-reperfusion injury in rats[J]. Chin Exp Surg, 2020, 37(10): 1826-1829. DOI: 10.3760/cma.j.cn421213-20191031-00772.金丽明, 黄东胜, 刘原兴, 等. 鞘氨醇激酶及1-磷酸鞘氨醇信号参与大鼠肝窦微循环保护[J]. 中华实验外科杂志, 2020, 37(10): 1826-1829. DOI: 10.3760/cma.j.cn421213-20191031-00772. [17] JIN LM, LIU YX, CHENG J, et al. The effect of SphK1/S1P signaling pathway on hepatic sinus microcirculation in rats with hepatic ischemia-reperfusion injury[J]. Hepatobiliary Pancreat Dis Int, 2022, 21(1): 94-98. DOI: 10.1016/j.hbpd.2021.06.003. [18] HUANG LS, SUDHADEVI T, FU P, et al. Sphingosine kinase 1/S1P signaling contributes to pulmonary fibrosis by activating Hippo/YAP pathway and mitochondrial reactive oxygen species in lung fibroblasts[J]. Int J Mol Sci, 2020, 21(6): 2064. DOI: 10.3390/ijms21062064. [19] KONO Y, NISHIUMA T, NISHIMURA Y, et al. Sphingosine kinase 1 regulates differentiation of human and mouse lung fibroblasts mediated by TGF-beta1[J]. Am J Respir Cell Mol Biol, 2007, 37(4): 395-404. DOI: 10.1165/rcmb.2007-0065OC. [20] CENCETTI F, BERNACCHIONI C, NINCHERI P, et al. Transforming growth factor-beta1 induces transdifferentiation of myoblasts into myofibroblasts via up-regulation of sphingosine kinase-1/S1P3 axis[J]. Mol Biol Cell, 2010, 21(6): 1111-1124. DOI: 10.1091/mbc.e09-09-0812. [21] LIAO YS, BAI LL. Role of exercise-induced autophagy in prevention and treatment of nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2022, 38(5): 1156-1160. DOI: 10.3969/j.issn.1001-5256.2022.05.038.廖粤生, 白莉莉. 运动诱导细胞自噬在非酒精性脂肪性肝病防治中的作用[J]. 临床肝胆病杂志, 2022, 38(5): 1156-1160. DOI: 10.3969/j.issn.1001-5256.2022.05.038. [22] LIU GJ, PENG L. Study on the role of autophagy in hepatic fibrosis[J]. Shandong Med J, 2018, 58(48): 108-110. DOI: 10.3969/j.issn.1002-266X.2018.48.031.刘国菊, 彭雷. 自噬在肝纤维化中作用的研究进展[J]. 山东医药, 2018, 58(48): 108-110. DOI: 10.3969/j.issn.1002-266X.2018.48.031. [23] WANG X, WU R, LIU Y, et al. m6A mRNA methylation controls autophagy and adipogenesis by targeting Atg5 and Atg7[J]. Autophagy, 2020, 16(7): 1221-1235. DOI: 10.1080/15548627.2019.1659617. [24] KURAMOTO K, KIM YJ, HONG JH, et al. The autophagy protein Becn1 improves insulin sensitivity by promoting adiponectin secretion via exocyst binding[J]. Cell Rep, 2021, 35(8): 109184. DOI: 10.1016/j.celrep.2021.109184. [25] ALEXAKI A, GUPTA SD, MAJUMDER S, et al. Autophagy regulates sphingolipid levels in the liver[J]. J Lipid Res, 2014, 55(12): 2521-2531. DOI: 10.1194/jlr.M051862. -



 PDF下载 ( 4489 KB)
PDF下载 ( 4489 KB)


 下载:
下载: