肠道菌群在慢加急性肝衰竭中的变化特征及致病机制
DOI: 10.3969/j.issn.1001-5256.2023.08.034
利益冲突声明: 本文不存在任何利益冲突。
作者贡献声明: 陈桂容、王明刚、王秀峰对文章的思路及设计有关键贡献;陈桂容负责拟定写作框架并撰写文章;林华明、严惠萍均参与了文献检索及论文修订;王明刚、王秀峰负责指导修改并最终定稿。
Changes and pathogenic mechanism of intestinal flora in acute-on-chronic liver failure
-
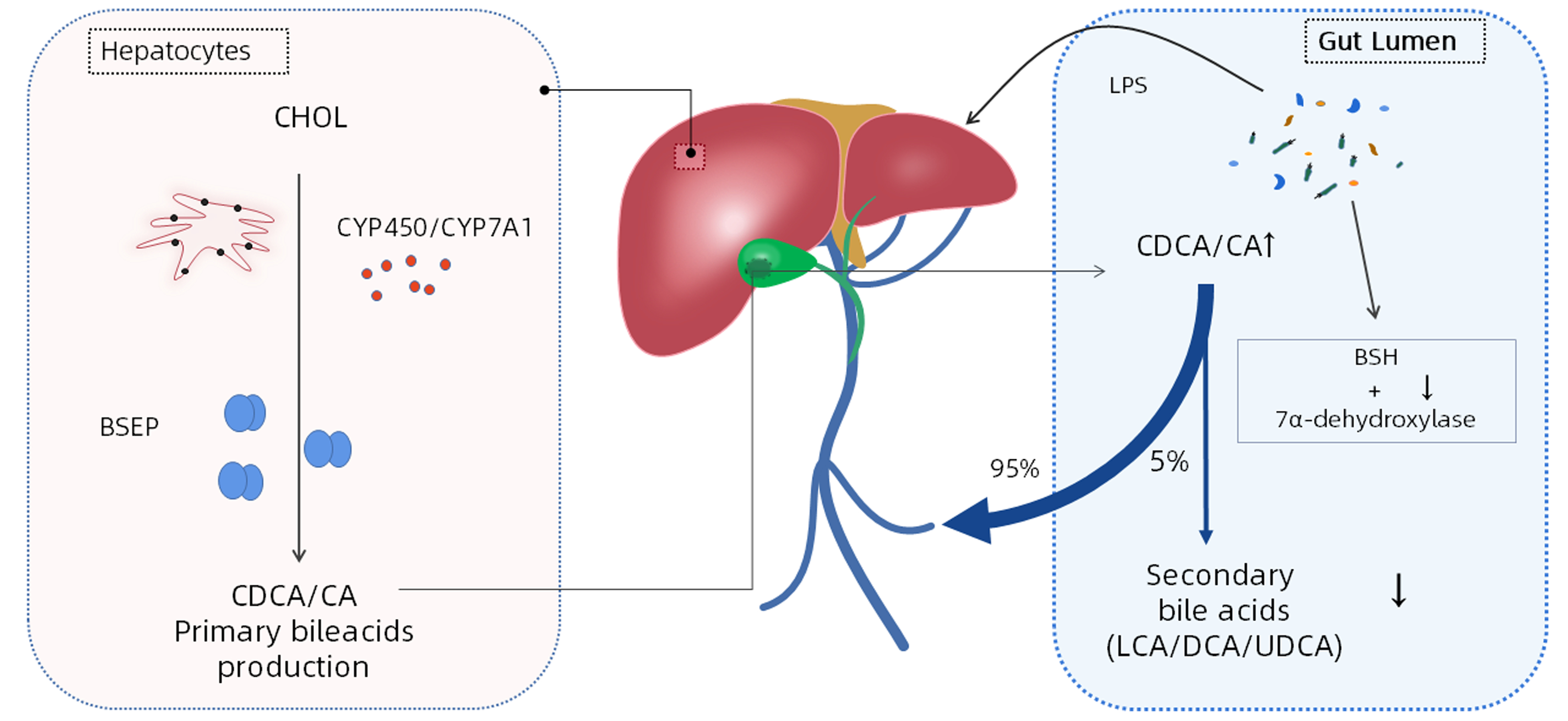
摘要: 慢加急性肝衰竭起病迅速而病死率高,预后差,且缺乏特异性药物治疗及手段。近年来,越来越多的证据表明肠道微生物群对维持人体微环境稳态的作用至关重要。利用宏基因组学全面测试肠道菌群特征可证明肠道菌群与慢性肝脏疾病发生发展方面存在交互关联。在慢加急性肝衰竭发病机制研究中,发现肠道微生物群在其致病过程中扮演重要角色。基于此,本文总结了慢加急性肝衰竭发生发展过程中肠道菌群的变化特征及其参与致病的机制途径,以期从肠道菌群调控新视角为慢加急性肝衰竭的临床治疗提供新靶点。Abstract: Acute-on-chronic liver failure (ACLF) has a rapid onset, a high mortality rate, and a poor prognosis, and there is still a lack of specific pharmacotherapy and treatment methods. In recent years, an increasing number of evidence has shown that intestinal flora plays a critical role in maintaining the homeostasis of human microenvironment. Comprehensive testing of intestinal flora profile using metagenomics has demonstrated an interaction association between intestinal flora and the development and progression of chronic liver diseases. Research on the pathogenesis of ACLF has found that intestinal flora plays an important role in the pathogenesis of ACLF. Based on this, this article summarizes the changes in intestinal flora and its pathogenic mechanism in the development and progression of ACLF, so as to provide new targets for the clinical treatment of ACLF from the new perspective of intestinal flora regulation.
-
-
[1] MEZZANO G, JUANOLA A, CARDENAS A, et al. Global burden of disease: acute-on-chronic liver failure, a systematic review and meta-analysis[J]. Gut, 2022, 71(1): 148-155. DOI: 10.1136/gutjnl-2020-322161. [2] GIANNELLI V, DI GREGORIO V, IEBBA V, et al. Microbiota and the gut-liver axis: bacterial translocation, inflammation and infection in cirrhosis[J]. World J Gastroenterol, 2014, 20(45): 16795-16810. DOI: 10.3748/wjg.v20.i45.16795. [3] GOMAA EZ. Human gut microbiota/microbiome in health and diseases: a review[J]. Antonie Van Leeuwenhoek, 2020, 113(12): 2019-2040. DOI: 10.1007/s10482-020-01474-7. [4] LAN P, YIN SM, HE Z. Research progress of gut microbiota in the prevention and treatment of colorectal cancer[J]. Chin J Dig Surg, 2022, 21(6): 730-736. DOI: 10.3760/cma.j.cn115610-20220402-00176.兰平, 殷盛梅, 何真. 肠道微生态在结直肠癌预防和治疗中的研究进展[J]. 中华消化外科杂志, 2022, 21(6): 730-736. DOI: 10.3760/cma.j.cn115610-20220402-00176. [5] O'HARA AM, SHANAHAN F. The gut flora as a forgotten organ[J]. EMBO Rep, 2006, 7(7): 688-693. DOI: 10.1038/sj.embor.7400731. [6] FAN Y, PEDERSEN O. Gut microbiota in human metabolic health and disease[J]. Nat Rev Microbiol, 2021, 19(1): 55-71. DOI: 10.1038/s41579-020-0433-9. [7] CHOPYK DM, GRAKOUI A. Contribution of the Intestinal microbiome and gut barrier to hepatic disorders[J]. Gastroenterology, 2020, 159(3): 849-863. DOI: 10.1053/j.gastro.2020.04.077. [8] HIIPPALA K, JOUHTEN H, RONKAINEN A, et al. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation[J]. Nutrients, 2018, 10(8): 988. DOI: 10.3390/nu10080988. [9] TREBICKA J, BORK P, KRAG A, et al. Utilizing the gut microbiome in decompensated cirrhosis and acute-on-chronic liver failure[J]. Nat Rev Gastroenterol Hepatol, 2021, 18(3): 167-180. DOI: 10.1038/s41575-020-00376-3. [10] ZHANG Y, ZHANG J, XU T, et al. Allicin ameliorates intraintestinal bacterial translocation after trauma/hemorrhagic shock in rats: The role of mesenteric lymph node dendritic cell[J]. Surgery, 2017, 161(2): 546-555. DOI: 10.1016/j.surg.2016.08.029. [11] HU Z, WU YS. The role and mechanism of gut microbiota in the occurrence of colorectal cancer[J]. Chin J Dig Surg, 2021, 20(6): 708-712. DOI: 10.3760/cma.j.cn115610-20210323-00142.胡洲, 武永胜. 肠道菌群在结直肠癌发生中的作用及机制[J]. 中华消化外科杂志, 2021, 20(6): 708-712. DOI: 10.3760/cma.j.cn115610-20210323-00142. [12] MEIR M, FLEMMING S, BURKARD N, et al. The glial cell-line derived neurotrophic factor: a novel regulator of intestinal barrier function in health and disease[J]. Am J Physiol Gastrointest Liver Physiol, 2016, 310(11): G1118-G1123. DOI: 10.1152/ajpgi.00125.2016. [13] MACIA L, TAN J, VIEIRA AT, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome[J]. Nat Commun, 2015, 6: 6734. DOI: 10.1038/ncomms7734. [14] KIM SE, PARK JW, KIM HS, et al. The role of gut dysbiosis in acute-on-chronic liver failure[J]. Int J Mol Sci, 2021, 22(21): 11680. DOI: 10.3390/ijms222111680. [15] BAJAJ JS, VARGAS HE, REDDY KR, et al. Association between intestinal microbiota collected at hospital admission and outcomes of patients with cirrhosis[J]. Clin Gastroenterol Hepatol, 2019, 17(4): 756-765.e3. DOI: 10.1016/j.cgh.2018.07.022. [16] BAJAJ JS, HEUMAN DM, HYLEMON PB, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications[J]. J Hepatol, 2014, 60(5): 940-947. DOI: 10.1016/j.jhep.2013.12.019. [17] KAKIYAMA G, PANDAK WM, GILLEVET PM, et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis[J]. J Hepatol, 2013, 58(5): 949-955. DOI: 10.1016/j.jhep.2013.01.003. [18] FERNÁNDEZ J, ACEVEDO J, WIEST R, et al. European Foundation for the Study of Chronic Liver Failure. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis[J]. Gut, 2018, 67(10): 1870-1880. DOI: 10.1136/gutjnl-2017-314240. [19] JENNE CN, KUBES P. Immune surveillance by the liver[J]. Nat Immunol, 2013, 14(10): 996-1006. DOI: 10.1038/ni.2691. [20] ARVANITI V, D'AMICO G, FEDE G, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis[J]. Gastroenterology, 2010, 139(4): 1246-1256, 1256.e1-5. DOI: 10.1053/j.gastro.2010.06.019. [21] MÜCKE MM, RUMYANTSEVA T, MÜCKE VT, et al. Bacterial infection-triggered acute-on-chronic liver failure is associated with increased mortality[J]. Liver Int, 2018, 38(4): 645-653. DOI: 10.1111/liv.13568. [22] BRUNS T, REUKEN PA, STENGEL S, et al. The prognostic significance of bacterial DNA in patients with decompensated cirrhosis and suspected infection[J]. Liver Int, 2016, 36(8): 1133-1142. DOI: 10.1111/liv.13095. [23] LI CQ, GUO JC, XUN YH, et al. Characterization of intestinal flora in chronic plus acute liver failure combined with spontaneous peritonitis[J]. Zhejiang J Integr Tradit Chin West Med, 2021, 31(11): 1012-1015. DOI: 10.3969/j.issn.1005-4561.2021.11.007.李春青, 过建春, 荀运浩, 等. 慢加急性肝衰竭合并自发性腹膜炎肠道菌群特征分析[J]. 浙江中西医结合杂志, 2021, 31(11): 1012-1015. DOI: 10.3969/j.issn.1005-4561.2021.11.007. [24] ALEXOPOULOU A, VASILIEVA L, AGIASOTELLI D, et al. Extensively drug-resistant bacteria are an independent predictive factor of mortality in 130 patients with spontaneous bacterial peritonitis or spontaneous bacteremia[J]. World J Gastroenterol, 2016, 22(15): 4049-4056. DOI: 10.3748/wjg.v22.i15.4049. [25] ZHANG W, WANG GC, ZHANG T, et al. Distribution characteristics and drug resistance analysis of clinically isolated pathogens in patients complicated with decompensated hepatitis B cirrhosis and spontaneous bacterial peritonitis[J]. Int J Lab Med, 2022, 43(19): 2346-2351. DOI: 10.3969/j.issn.1673-4130.2022.19.009.张旺, 王国充, 张甜, 等. 乙型肝炎肝硬化失代偿期并发自发性细菌性腹膜炎患者临床分离菌的分布特点及耐药情况分析[J]. 国际检验医学杂志, 2022, 43(19): 2346-2351. DOI: 10.3969/j.issn.1673-4130.2022.19.009. [26] ENGELMANN C, SHEIKH M, SHARMA S, et al. Toll-like receptor 4 is a therapeutic target for prevention and treatment of liver failure[J]. J Hepatol, 2020, 73(1): 102-112. DOI: 10.1016/j.jhep.2020.01.011. [27] WANG Y, CHEN H, CHEN Q, et al. The protective mechanism of CAY10683 on intestinal mucosal barrier in acute liver failure through LPS/TLR4/MyD88 pathway[J]. Mediators Inflamm, 2018, 2018: 7859601. DOI: 10.1155/2018/7859601. [28] BIAGIOLI M, CARINO A. Signaling from intestine to the host: how bile acids regulate intestinal and liver immunity[J]. Handb Exp Pharmacol, 2019, 256: 95-108. DOI: 10.1007/164_2019_225. [29] GRÜNER N, MATTNER J. Bile acids and microbiota: multifaceted and versatile regulators of the liver-gut axis[J]. Int J Mol Sci, 2021, 22(3): 1397. DOI: 10.3390/ijms22031397. [30] PÉAN N, DOIGNON I, GARCIN I, et al. The receptor TGR5 protects the liver from bile acid overload during liver regeneration in mice[J]. Hepatology, 2013, 58(4): 1451-1460. DOI: 10.1002/hep.26463. [31] CICHOŻ-LACH H, MICHALAK A. Oxidative stress as a crucial factor in liver diseases[J]. World J Gastroenterol, 2014, 20(25): 8082-8091. DOI: 10.3748/wjg.v20.i25.8082. [32] ZHONG W, QIAN K, XIONG J, et al. Curcumin alleviates lipopolysaccharide induced sepsis and liver failure by suppression of oxidative stress-related inflammation via PI3K/AKT and NF-κB related signaling[J]. Biomed Pharmacother, 2016, 83: 302-313. DOI: 10.1016/j.biopha.2016.06.036. [33] POPA GL, POPA MI. Oxidative stress in chronic hepatitis B-an update[J]. Microorganisms, 2022, 10(7): 1265. DOI: 10.3390/microorganisms10071265. [34] GUSTOT T, LEMMERS A, MORENO C, et al. Differential liver sensitization to toll-like receptor pathways in mice with alcoholic fatty liver[J]. Hepatology, 2006, 43(5): 989-1000. DOI: 10.1002/hep.21138. [35] BEAM A, CLINGER E, HAO L. Effect of diet and dietary components on the composition of the gut microbiota[J]. Nutrients, 2021, 13(8): 2795. DOI: 10.3390/nu13082795. [36] SO D, WHELAN K, ROSSI M, et al. Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis[J]. Am J Clin Nutr, 2018, 107(6): 965-983. DOI: 10.1093/ajcn/nqy041. [37] KOH A, de VADDER F, KOVATCHEVA-DATCHARY P, et al. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites[J]. Cell, 2016, 165(6): 1332-1345. DOI: 10.1016/j.cell.2016.05.041. [38] KOCOT AM, JAROCKA-CYRTA E, DRABIŃSKA N. Overview of the importance of biotics in gut barrier integrity[J]. Int J Mol Sci, 2022, 23(5): 2896. DOI: 10.3390/ijms23052896. [39] CONG J, ZHOU P, ZHANG R. Intestinal microbiota-derived short chain fatty acids in host health and disease[J]. Nutrients, 2022, 14(9): 1977. DOI: 10.3390/nu14091977. [40] HORVATH A, DURDEVIC M, LEBER B, et al. Changes in the intestinal microbiome during a multispecies probiotic intervention in compensated cirrhosis[J]. Nutrients, 2020, 12(6): 1874. DOI: 10.3390/nu12061874. [41] PATEL VC, LEE S, MCPHAIL M, et al. Rifaximin-α reduces gut-derived inflammation and mucin degradation in cirrhosis and encephalopathy: RIFSYS randomised controlled trial[J]. J Hepatol, 2022, 76(2): 332-342. DOI: 10.1016/j.jhep.2021.09.010. [42] BECATTINI S, TAUR Y, PAMER EG. Antibiotic-induced changes in the intestinal microbiota and disease[J]. Trends Mol Med, 2016, 22(6): 458-478. DOI: 10.1016/j.molmed.2016.04.003. -


 PDF下载 ( 2330 KB)
PDF下载 ( 2330 KB)


 下载:
下载: