热休克蛋白90在HBV复制中的作用
DOI: 10.3969/j.issn.1001-5256.2023.10.023
-
摘要: HBV传播范围广,慢性感染状态多,治愈率低,提高HBV治愈率有助于改善患者的长期预后状况。热休克蛋白90(Hsp90)是广泛存在于生物体内的一种分子伴侣蛋白。近年来越来越多的研究表明,Hsp90与HBV感染有关,在HBV的复制过程中起重要作用,可与病毒的特定蛋白相互作用促进病毒复制,也可与宿主自身蛋白相互作用来发挥功能。本文就近年来Hsp90在HBV复制中的作用相关研究进展作一综述,以期为以Hsp90为靶点的抗HBV药物研发与HBV防治提供新的理论指导和方向。
-
关键词:
- 乙型肝炎病毒 /
- HSP90热休克蛋白质类 /
- 病毒复制
Abstract: Hepatitis B virus (HBV) has the characteristics of wide transmission, a high chronic infection rate, and a low cure rate, and improving the cure rate of HBV may help to improve the long-term prognosis of patients. Heat shock protein 90 (Hsp90) is a chaperone protein widely present in organisms. In recent years, more and more studies have shown that Hsp90 is associated with HBV infection and plays an important role in HBV replication. It can not only interact with specific proteins of the virus to promote its replication, but also interact with the host’s own proteins to perform its function. This article reviews the role of Hsp90 in HBV replication in recent studies, so as to provide new theoretical guidance and directions for the development of new anti-HBV drugs targeting Hsp90 and the prevention and treatment of HBV infection in the future.-
Key words:
- Hepatitis B Virus /
- HSP90 Heat-Shock Proteins /
- Virus Replication
-
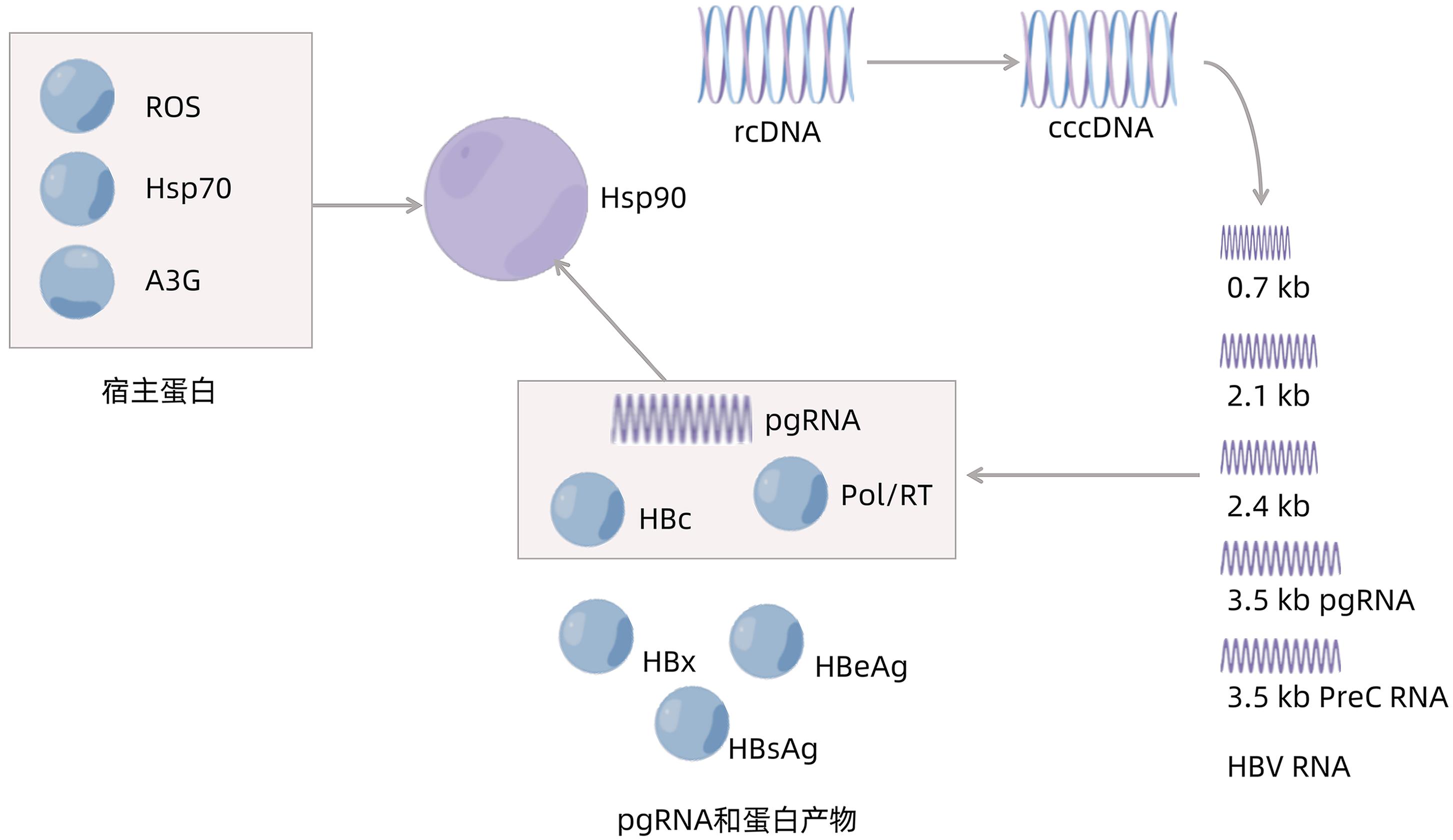
图 1 HBV复制过程示意图
注: HBV复制过程起始于rcDNA,rcDNA转化为cccDNA,其转录产物RNA再进一步翻译为蛋白质Pol/RT、HBx、HBeAg、HBsAg、HBc。其中pgRNA、HBc和Pol/RT能够与Hsp90相互作用。此外,宿主蛋白Hsp70、活性氧(reactive oxygen species,ROS)和载脂蛋白B mRNA编辑酶催化多肽3G(apolipoprotein B mRNA editing enzyme catalytic polypeptide-like 3G,A3G)也能够与Hsp90相互作用。
Figure 1. Diagram of HBV replication process
-
[1] CHEN J, HUANG AL. Functional cure of hepatitis B from the perspective of hepatitis B virus covalently closed circular DNA[J]. J Clin Hepatol, 2022, 38( 8): 1716- 1720. DOI: 10.3969/j.issn.1001-5256.2022.08.003.陈娟, 黄爱龙. 从HBV cccDNA角度谈乙型肝炎的功能性治愈[J]. 临床肝胆病杂志, 2022, 38( 8): 1716- 1720. DOI: 10.3969/j.issn.1001-5256.2022.08.003. [2] BUCHNER J. Molecular chaperones and protein quality control: an introduction to the JBC Reviews thematic series[J]. J Biol Chem, 2019, 294( 6): 2074- 2075. DOI: 10.1074/jbc.REV118.006739. [3] HU C, YANG J, QI Z, et al. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities[J]. MedComm(2020), 2022, 3( 3): e161. DOI: 10.1002/mco2.161. [4] LANG BJ, PRINCE TL, OKUSHA Y, et al. Heat shock proteins in cell signaling and cancer[J]. Biochim Biophys Acta Mol Cell Res, 2022, 1869( 3): 119187. DOI: 10.1016/j.bbamcr.2021.119187. [5] JAKOB U, LILIE H, MEYER I, et al. Transient interaction of Hsp90 with early unfolding intermediates of citrate synthase. Implications for heat shock in vivo[J]. J Biol Chem, 1995, 270( 13): 7288- 7294. DOI: 10.1074/jbc.270.13.7288. [6] WIECH H, BUCHNER J, ZIMMERMANN R, et al. Hsp90 chaperones protein folding in vitro[J]. Nature, 1992, 358( 6382): 169- 170. DOI: 10.1038/358169a0. [7] CASTRO JP, FERNANDO R, REEG S, et al. Non-enzymatic cleavage of Hsp90 by oxidative stress leads to actin aggregate formation: A novel gain-of-function mechanism[J]. Redox Biol, 2019, 21: 101108. DOI: 10.1016/j.redox.2019.101108. [8] KIM MJ, KIM J, IM JS, et al. Hepatitis B virus X protein enhances liver cancer cell migration by regulating calmodulin-associated actin polymerization[J]. BMB Rep, 2021, 54( 12): 614- 619. DOI: 10.5483/BMBRep.2021.54.12.084. [9] WEIS F, MOULLINTRAFFORT L, HEICHETTE C, et al. The 90-kDa heat shock protein Hsp90 protects tubulin against thermal denaturation[J]. J Biol Chem, 2010, 285( 13): 9525- 9534. DOI: 10.1074/jbc.M109.096586. [10] KRTKOVÁ J, ZIMMERMANN A, SCHWARZEROVÁ K, et al. Hsp90 binds microtubules and is involved in the reorganization of the microtubular network in angiosperms[J]. J Plant Physiol, 2012, 169( 14): 1329- 1339. DOI: 10.1016/j.jplph.2012.06.010. [11] HALLETT ST, PASTOK MW, MORGAN R, et al. Differential regulation of G1 CDK complexes by the Hsp90-Cdc37 chaperone system[J]. Cell Rep, 2017, 21( 5): 1386- 1398. DOI: 10.1016/j.celrep.2017.10.042. [12] MIKOLAJCZYK M, NELSON MA. Regulation of stability of cyclin-dependent kinase CDK11p110 and a caspase-processed form, CDK11p46, by Hsp90[J]. Biochem J, 2004, 384( Pt 3): 461- 467. DOI: 10.1042/BJ20040848. [13] ALIGUE R, AKHAVAN-NIAK H, RUSSELL P. A role for Hsp90 in cell cycle control: Wee1 tyrosine kinase activity requires interaction with Hsp90[J]. EMBO J, 1994, 13( 24): 6099- 6106. DOI: 10.1002/j.1460-2075.1994.tb06956.x. [14] MEGAHED F, ZHOU X, SUN P. The interactions between HBV and the innate immunity of hepatocytes[J]. Viruses, 2020, 12( 3): 285. DOI: 10.3390/v12030285. [15] SEEGER C, MASON WS. Hepatitis B virus biology[J]. Microbiol Mol Biol Rev, 2000, 64( 1): 51- 68. DOI: 10.1128/MMBR.64.1.51-68.2000. [16] SHIM HY, QUAN X, YI YS, et al. Heat shock protein 90 facilitates formation of the HBV capsid via interacting with the HBV core protein dimers[J]. Virology, 2011, 410( 1): 161- 169. DOI: 10.1016/j.virol.2010.11.005. [17] TAVERNITI V, LIGAT G, DEBING Y, et al. Capsid assembly modulators as antiviral agents against HBV: Molecular mechanisms and clinical perspectives[J]. J Clin Med, 2022, 11( 5): 1349. DOI: 10.3390/jcm11051349. [18] ZHAO Q, HU Z, CHENG J, et al. Hepatitis B virus core protein dephosphorylation occurs during pregenomic RNA encapsidation[J]. J Virol, 2018, 92( 13): e02139- 17. DOI: 10.1128/JVI.02139-17. [19] MAKOKHA GN, ABE-CHAYAMA H, CHOWDHURY S, et al. Regulation of the Hepatitis B virus replication and gene expression by the multi-functional protein TARDBP[J]. Sci Rep, 2019, 9( 1): 8462. DOI: 10.1038/s41598-019-44934-5. [20] ZOULIM F, SEEGER C. Reverse transcription in hepatitis B viruses is primed by a tyrosine residue of the polymerase[J]. J Virol, 1994, 68( 1): 6- 13. DOI: 10.1128/JVI.68.1.6-13.1994. [21] LIU XQ, OHSAKI E, UEDA K. Establishment of a system for finding inhibitors of ε RNA binding with the HBV polymerase[J]. Genes Cells, 2020, 25( 8): 523- 537. DOI: 10.1111/gtc.12778. [22] HU J, FLORES D, TOFT D, et al. Requirement of heat shock protein 90 for human hepatitis B virus reverse transcriptase function[J]. J Virol, 2004, 78( 23): 13122- 13131. DOI: 10.1128/JVI.78.23.13122-13131.2004. [23] TAJWAR R, BRADLEY DP, PONZAR NL, et al. Predicted structure of the hepatitis B virus polymerase reveals an ancient conserved protein fold[J]. Protein Sci, 2022, 31( 10): e4421. DOI: 10.1002/pro.4421. [24] GYOO PARK S, KYUNG RHO J, JUNG G. Hsp90 makes the human HBV Pol competent for in vitro priming rather than maintaining the human HBV Pol/pregenomic RNA complex[J]. Arch Biochem Biophys, 2002, 401( 1): 99- 107. DOI: 10.1016/S0003-9861(02)00004-8. [25] KIM YS, SEO HW, JUNG G. Reactive oxygen species promote heat shock protein 90-mediated HBV capsid assembly[J]. Biochem Biophys Res Commun, 2015, 457( 3): 328- 333. DOI: 10.1016/j.bbrc.2014.12.110. [26] LIM SO, PARK SG, YOO JH, et al. Expression of heat shock proteins(HSP27, HSP60, HSP70, HSP90, GRP78, GRP94) in hepatitis B virus-related hepatocellular carcinomas and dysplastic nodules[J]. World J Gastroenterol, 2005, 11( 14): 2072- 2079. DOI: 10.3748/wjg.v11.i14.2072. [27] SEO HW, SEO JP, JUNG G. Heat shock protein 70 and heat shock protein 90 synergistically increase hepatitis B viral capsid assembly[J]. Biochem Biophys Res Commun, 2018, 503( 4): 2892- 2898. DOI: 10.1016/j.bbrc.2018.08.065. [28] POPA GL, POPA MI. Oxidative stress in chronic hepatitis B-an update[J]. Microorganisms, 2022, 10( 7): 1265. DOI: 10.3390/microorganisms10071265. [29] TURELLI P, MANGEAT B, JOST S, et al. Inhibition of hepatitis B virus replication by APOBEC3G[J]. Science, 2004, 303( 5665): 1829. DOI: 10.1126/science.1092066. [30] NGUYEN DH, HU J. Reverse transcriptase- and RNA packaging signal-dependent incorporation of APOBEC3G into hepatitis B virus nucleocapsids[J]. J Virol, 2008, 82( 14): 6852- 6861. DOI: 10.1128/JVI.00465-08. [31] CHEN Z, EGGERMAN TL, BOCHAROV AV, et al. Heat shock proteins stimulate APOBEC-3-mediated cytidine deamination in the hepatitis B virus[J]. J Biol Chem, 2017, 292( 32): 13459- 13479. DOI: 10.1074/jbc.M116.760637. [32] MITRA B, THAPA RJ, GUO H, et al. Host functions used by hepatitis B virus to complete its life cycle: Implications for developing host-targeting agents to treat chronic hepatitis B[J]. Antiviral Res, 2018, 158: 185- 198. DOI: 10.1016/j.antiviral.2018.08.014. [33] BROUGH PA, AHERNE W, BARRIL X, et al. 4,5-diarylisoxazole Hsp90 chaperone inhibitors: potential therapeutic agents for the treatment of cancer[J]. J Med Chem, 2008, 51( 2): 196- 218. DOI: 10.1021/jm701018h. [34] HU KH, HUANG YY, MU JF, et al. KNK437 inhibits replication and transcription of the hepatitis B virus[J]. Chin J Virol, 2017, 33( 1): 24- 35. DOI: 10.13242/j.cnki.bingduxuebao.003090.胡康洪, 黄亚运, 穆敬芳, 等. KNK437抑制乙型肝炎病毒复制及转录[J]. 病毒学报, 2017, 33( 1): 24- 35. DOI: 10.13242/j.cnki.bingduxuebao.003090. [35] HU L, WANG Y, CHEN Z, et al. Hsp90 inhibitor SNX-2112 enhances TRAIL-induced apoptosis of human cervical cancer cells via the ROS-mediated JNK-p53-autophagy-DR5 pathway[J]. Oxid Med Cell Longev, 2019, 2019: 9675450. DOI: 10.1155/2019/9675450. [36] GUAN L, ZOU Q, LIU Q, et al. HSP90 inhibitor ganetespib(STA-9090) inhibits tumor growth in c-Myc-dependent esophageal squamous cell carcinoma[J]. Onco Targets Ther, 2020, 13: 2997- 3011. DOI: 10.2147/OTT.S245813. -



 PDF下载 ( 630 KB)
PDF下载 ( 630 KB)

 下载:
下载:


