无法手术切除的胆管细胞癌经体部立体定向放射治疗的效果及安全性分析
DOI: 10.3969/j.issn.1001-5256.2023.11.021
Efficacy and safety of stereotactic body radiation therapy in treatment of patients with unresectable cholangiocarcinoma
-
摘要:
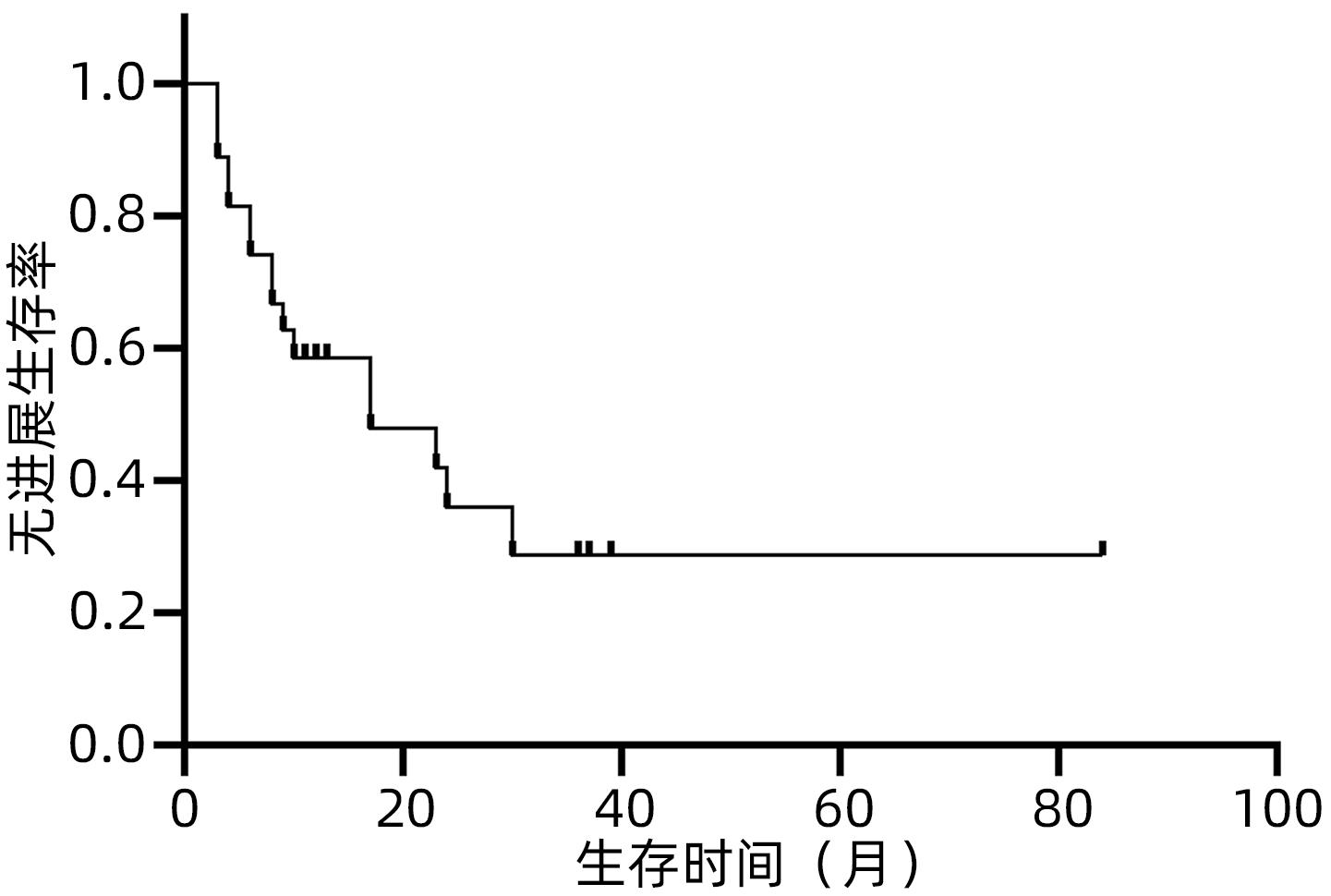
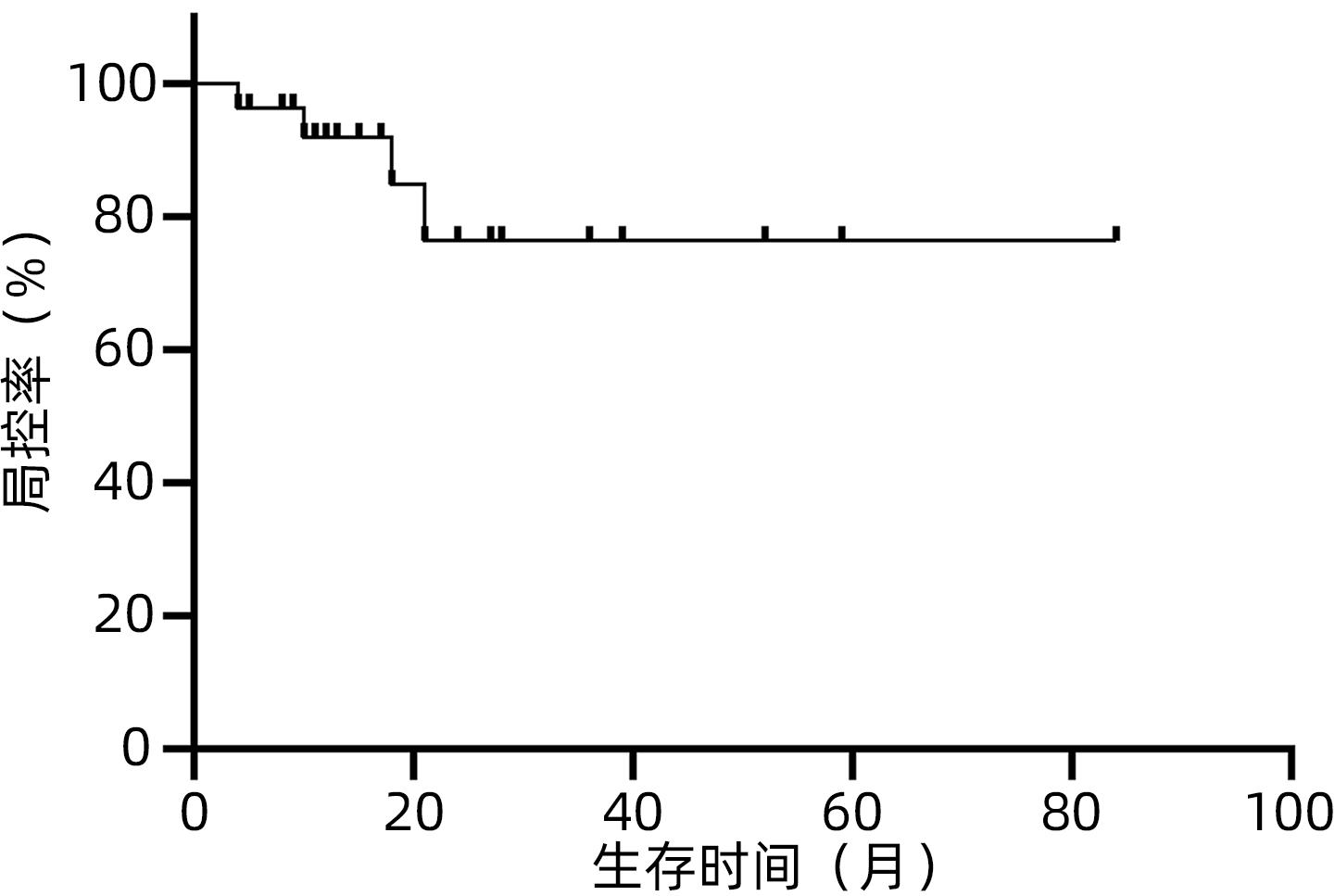
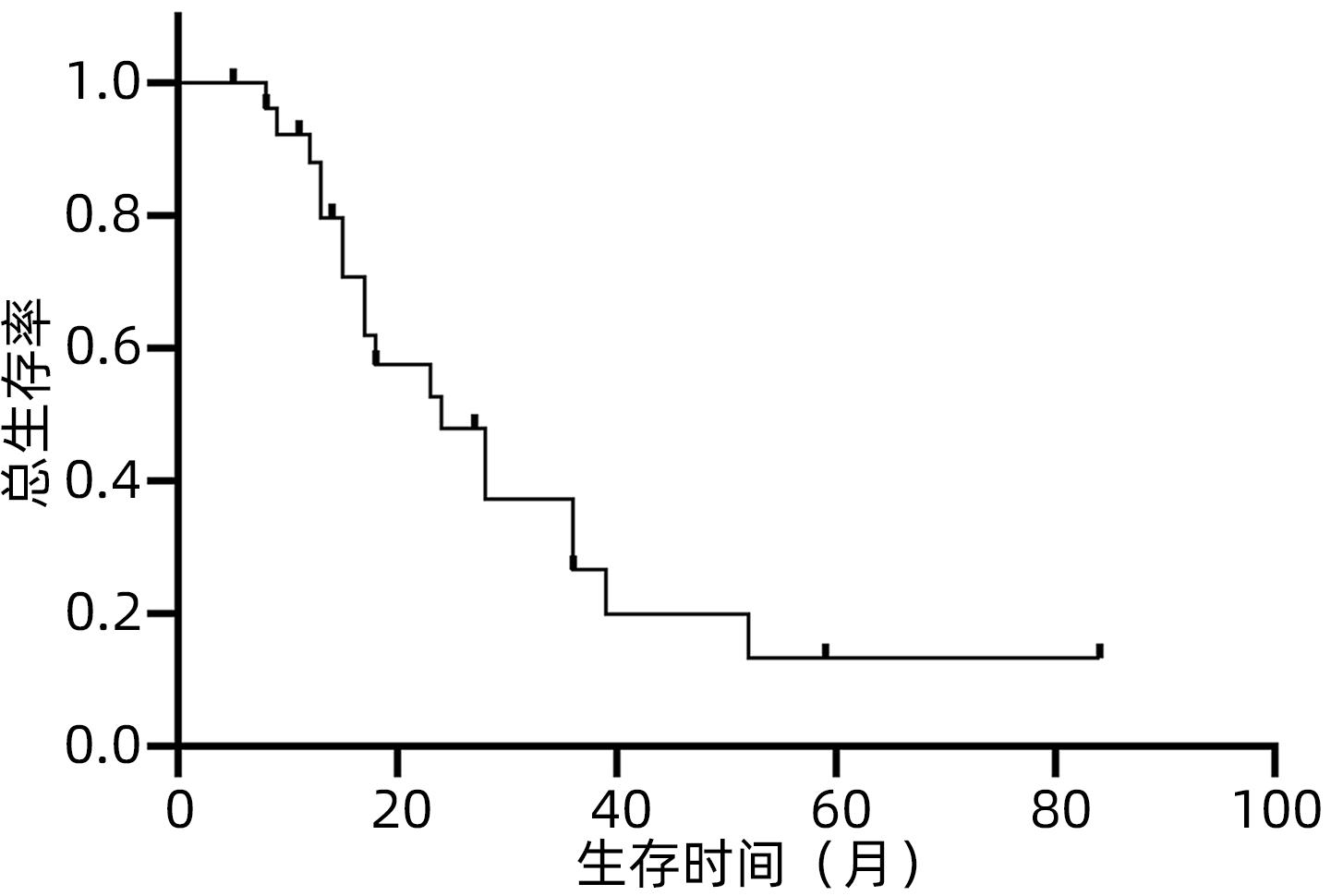
目的 观察体部立体定向放疗治疗无法手术切除的胆管细胞癌患者的生存情况及不良反应。 方法 选取2012年2月—2020年7月于解放军总医院第五医学中心行体部立体定向放射治疗的27例无法手术切除的单发无转移的胆管细胞癌患者。计划靶区(PTV)处方剂量42~60 Gy,分5~8次,5~11 Gy/次。其中有5例联合肝动脉化疗栓塞术(TACE)和化疗治疗。以6个月、12个月、18个月和24个月的总生存率、无进展生存率和局部控制率作为疗效评价指标。使用不良事件通用术语标准(CTCAE)v.4.03评估不良反应。采用Kaplan-Meier法计算总生存率、无进展生存率和局部控制率。 结果 中位随访时间17个月。27例患者的6个月、12个月、18个月和24个月的总生存率分别为100%、88%、57.5%和47.9%,无进展生存率分别为74.1%、58.6%、47.9%和35.9%,局部控制率分别为96.3%、91.9%、84.8%和76.4%。未出现3级及以上毒性反应。5例患者诊断为放射性肝损伤,无因放射性肝损伤死亡病例。 结论 体部立体定向放疗治疗不可切除胆管细胞癌安全、有效,具有较高的生存率、无进展生存率和局部控制率,且毒性反应低,可作为不适合手术治疗的胆管癌的替代治疗。 Abstract:Objective To investigate the survival and adverse reactions of patients with unresectable cholangiocarcinoma after stereotactic body radiation therapy (SBRT). Methods A total of 27 patients with unresectable solitary cholangiocarcinoma without metastasis who underwent SBRT in The Fifth Medical Center of Chinese PLA General Hospital from February 2012 to July 2020 were enrolled. The prescribed dose to planning target volume was 42-60 Gy in 5-8 fractions, with 5-11 Gy/fraction. Among these patients, five patients were also treated with chemotherapy and transcatheter arterial chemoembolization. The 6-, 12-, 18-, and 24-month overall survival (OS) rates, progression-free survival (PFS) rates, and local control (LC) rates were used as the assessment indices for treatment outcome; Common Terminology Criteria for Adverse Events v.4.03 was used to evaluate adverse reactions; the Kaplan-Meier method was used to calculate OS, PFS, and LC rates. Results The median follow-up time was 17 months. For all 27 patients, the 6-, 12-, 18-, and 24-month OS rates were 100%, 88%, 57.5%, and 47.9%, respectively; the 6-, 12-, 18-, and 24-month PFS rates were 74.1%, 58.6%, 47.9%, and 35.9%, respectively; the 6-, 12-, 18-, and 24-month LC rates were 96.3%, 91.9%, 84.8%, and 76.4%, respectively. No grade 3 or above toxic reactions were observed. Five patients were diagnosed with radiation-induced liver injury, but there was no death due to radiation-induced liver injury. Conclusion SBRT is safe and effective in the treatment of unresectable cholangiocarcinoma, with relatively high survival rate, PFS rate, and LC rate and low toxicity, and therefore, SBRT can be used as an alternative treatment method for patients with cholangiocarcinoma who are not candidates for surgery. -
Key words:
- Cholangiocarcinoma /
- Radiosurgery /
- Treatment Outcome
-
表 1 27例患者一般临床资料
Table 1. The characteristics of 27 patients
特征 数值 性别[例(%)] 男 19(70.4) 女 8(29.6) 年龄(岁) 64(52~68) 肿瘤位置[例(%)] 肝内 14(51.9) 肝门 13(48.1) 肿瘤大小(cm) 4.5(2.6~5.1) 肿瘤标志物 AFP(ng/mL) 2.85(1.51~5.80) CA19-9(U/mL) 129.65(28.69~521.98) 合并梗阻性黄疸[例(%)] 有 15(55.6) 无 12(44.4) ECOG PS评分[例(%)] 0分 5(18.5) 1分 22(81.5) Child-Pugh分级[例(%)] A级 15(55.6) B级 12(44.4) 治疗方案[例(%)] 单用SBRT 22(81.5) SBRT+替吉奥 4(14.8) SBRT+TACE+替吉奥 1(3.7) 放疗计划 总剂量(Gy) 50(42~60) 单次剂量(Gy) 10(5~11) 分割次数(次) 5(5~8) BED 83.0(71.4~115.0) 注:BED,生物有效剂量。 -
[1] BERTUCCIO P, MALVEZZI M, CARIOLI G, et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma[J]. J Hepatol, 2019, 71( 1): 104- 114. DOI: 10.1016/j.jhep.2019.03.013. [2] BANALES JM, MARIN J, LAMARCA A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management[J]. Nat Rev Gastroenterol Hepatol, 2020, 17( 9): 557- 588. DOI: 10.1038/s41575-020-0310-z. [3] HEWITT DB, BROWN ZJ, PAWLIK TM. Current perspectives on the surgical management of perihilar cholangiocarcinoma[J]. Cancers(Basel), 2022, 14( 9): 2208. DOI: 10.3390/cancers14092208. [4] BRIDGEWATER JA, GOODMAN KA, KALYAN A, et al. Biliary tract cancer: Epidemiology, radiotherapy, and molecular profiling[J]. Am Soc Clin Oncol Educ Book, 2016, 35: e194- e203. DOI: 10.1200/EDBK_160831. [5] BENAVIDES M, ANTÓN A, GALLEGO J, et al. Biliary tract cancers: SEOM clinical guidelines[J]. Clin Transl Oncol, 2015, 17( 12): 982- 987. DOI: 10.1007/s12094-015-1436-2. [6] SAHA SK, ZHU AX, FUCHS CS, et al. Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise[J]. Oncologist, 2016, 21( 5): 594- 599. DOI: 10.1634/theoncologist.2015-0446. [7] NIU YJ, ZHA Y, LI SJ, et al. Analysis on prognosis related factors of patients with cholangiocarcinoma after radical resection and establishment of survival prediction model[J]. J Jilin Univ(Med Edit), 2022, 48( 4): 979- 987. DOI: 10.13481/j.1671-587X.2022041.牛英杰, 查勇, 李思嘉, 等. 胆管癌根治性切除术后患者预后相关因素分析和生存预测模型构建[J]. 吉林大学学报(医学版), 2022, 48( 4): 979- 987. DOI: 10.13481/j.1671-587X.20220418. [8] KHAN SA, DAVIDSON BR, GOLDIN RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update[J]. Gut, 2012, 61( 12): 1657- 1669. DOI: 10.1136/gutjnl-2011-301748. [9] KOZAK MM, TOESCA D, VON EYBEN R, et al. Stereotactic body radiation therapy for cholangiocarcinoma: optimizing locoregional control with elective nodal irradiation[J]. Adv Radiat Oncol, 2020, 5( 1): 77- 84. DOI: 10.1016/j.adro.2019.08.003. [10] FRAKULLI R, BUWENGE M, MACCHIA G, et al. Stereotactic body radiation therapy in cholangiocarcinoma: a systematic review[J]. Br J Radiol, 2019, 92( 1097): 20180688. DOI: 10.1259/bjr.20180688. [11] RAY CE EDWARDS Jr,, MITH MT, et al. Metaanalysis of survival, complications, and imaging response following chemotherapy-based transarterial therapy in patients with unresectable intrahepatic cholangiocarcinoma[J]. J Vasc Interv Radiol, 2013, 24( 8): 1218- 1226. DOI: 10.1016/j.jvir.2013.03.019. [12] KOAY EJ, ODISIO BC, JAVLE M, et al. Management of unresectable intrahepatic cholangiocarcinoma: how do we decide among the various liver-directed treatments?[J]. Hepatobiliary Surg Nutr, 2017, 6( 2): 105- 116. DOI: 10.21037/hbsn.2017.01.16. [13] CRANE CH, MACDONALD KO, VAUTHEY JN, et al. Limitations of conventional doses of chemoradiation for unresectable biliary cancer[J]. Int J Radiat Oncol Biol Phys, 2002, 53( 4): 969- 974. DOI: 10.1016/s0360-3016(02)02845-6. [14] BUJOLD A, MASSEY CA, KIM JJ, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma[J]. J Clin Oncol, 2013, 31( 13): 1631- 1639. DOI: 10.1200/JCO.2012.44.1659. [15] MAHADEVAN A, MIKSAD R, GOLDSTEIN M, et al. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer[J]. Int J Radiat Oncol Biol Phys, 2011, 81( 4): e615-622. DOI: 10.1016/j.ijrobp.2011.04.045. [16] MAHADEVAN A, JAIN S, GOLDSTEIN M, et al. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer[J]. Int J Radiat Oncol Biol Phys, 2010, 78( 3): 735- 742. DOI: 10.1016/j.ijrobp.2009.08.046. [17] SUN J, WANG Q, HONG ZX, et al. Stereotactic body radiotherapy versus hepatic resection for hepatocellular carcinoma(≤5 cm): a propensity score analysis[J]. Hepatol Int, 2020, 14( 5): 788- 797. DOI: 10.1007/s12072-020-10088-0. [18] BISELLO S, CAMILLETTI AC, BERTINI F, et al. Stereotactic radiotherapy in intrahepatic cholangiocarcinoma: A systematic review[J]. Mol Clin Oncol, 2021, 15( 2): 152. DOI: 10.3892/mco.2021.2314. [19] KOAY EJ, OWEN D, DAS P. Radiation-induced liver disease and modern radiotherapy[J]. Semin Radiat Oncol, 2018, 28( 4): 321- 331. DOI: 10.1016/j.semradonc.2018.06.007. [20] DEOLIVEIRA ML, CUNNINGHAM SC, CAMERON JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution[J]. Ann Surg, 2007, 245( 5): 755- 762. DOI: 10.1097/01.sla.0000251366.62632.d3. [21] Expert Group of the State Key Project on Infectious Diseaseson‘Novel Strategies of Comprehensive and IndividualizdSurgical Treatment of Viral Hepatitis-related Liver Cancer’ from the Ministry of Science and Technology of China. Chinese expert consensus on the surgical management ofintrahepatic cholangiocarcinoma(2020 edition)[J]. Chin J Dig Surg, 2021, 20( 1): 1- 15. DOI: 10.3760/cma.j.cn115610-20201211-00777.科技部传染病防治重大专项课题“病毒性肝炎相关肝癌外科综合治疗的个体化和新策略研究”专家组. 肝内胆管癌外科治疗中国专家共识(2020版)[J]. 中华消化外科杂志, 2021, 20( 1): 1- 15. DOI: 10.3760/cma.j.cn115610-20201211-00777. [22] REN B, GUO Q, YANG Y, et al. A meta-analysis of the efficacy of postoperative adjuvant radiotherapy versus no radiotherapy for extrahepatic cholangiocarcinoma and gallbladder carcinoma[J]. Radiat Oncol, 2020, 15( 1): 15. DOI: 10.1186/s13014-020-1459-x. [23] HONG TS, WO JY, YEAP BY, et al. Multi-institutional phase Ⅱ study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma[J]. J Clin Oncol, 2016, 34( 5): 460- 468. DOI: 10.1200/JCO.2015.64.2710. [24] POLISTINA FA, GUGLIELMI R, BAIOCCHI C, et al. Chemoradiation treatment with gemcitabine plus stereotactic body radiotherapy for unresectable, non-metastatic, locally advanced hilar cholangiocarcinoma. Results of a five year experience[J]. Radiother Oncol, 2011, 99( 2): 120- 123. DOI: 10.1016/j.radonc.2011.05.016. [25] PARK SS, DONG H, LIU X, et al. PD-1 restrains radiotherapy-induced abscopal effect[J]. Cancer Immunol Res, 2015, 3( 6): 610- 619. DOI: 10.1158/2326-6066.CIR-14-0138. [26] POSTOW MA, CALLAHAN MK, BARKER CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma[J]. N Engl J Med, 2012, 366( 10): 925- 931. DOI: 10.1056/NEJMoa1112824. [27] DENG L, LIANG H, BURNETTE B, et al. Radiation and anti-PD-L1 antibody combinatorial therapy induces T cell-mediated depletion of myeloid-derived suppressor cells and tumor regression[J]. Oncoimmunology, 2014, 3: e28499. DOI: 10.4161/onci.28499. [28] KREIDIEH M, ZEIDAN YH, SHAMSEDDINE A. The combination of stereotactic body radiation therapy and immunotherapy in primary liver tumors[J]. J Oncol, 2019, 2019: 4304817. DOI: 10.1155/2019/4304817. [29] BAO G, LIU H, MA Y, et al. The clinical efficacy and safety of different biliary drainages in malignant obstructive jaundice treatment[J]. Am J Transl Res, 2021, 13( 6): 7400- 7405. -



 PDF下载 ( 672 KB)
PDF下载 ( 672 KB)

 下载:
下载: