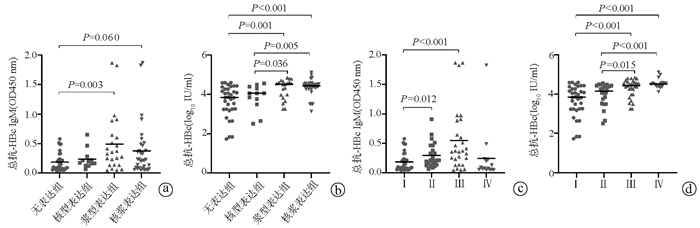
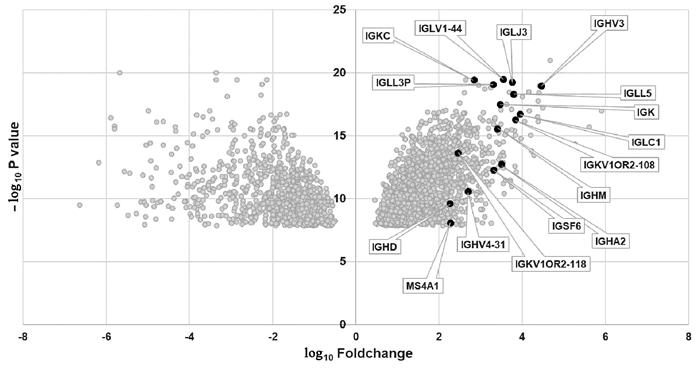
| [1] |
Chinese Society of Hepatology and Chinese Society of Infectious Diseases Chinese, Medical Association. The guideline of prevention and treatment for chronic hepatitis B: A 2015 update[J]. J Clin Hepatol, 2015, 31(12): 1941-1960. DOI: 10.3969/j.issn.1001-5256.2015.12.002. |
| [2] |
YEO YH, HO HJ, YANG HI, et al. Factors associated with rates of HBsAg seroclearance in adults with chronic HBV infection: A systematic review and Meta-analysis[J]. Gastroenterology, 2019, 156(3): 635-646. e9. DOI: 10.1053/j.gastro.2018.10.027. |
| [3] |
LOK AS, MCMAHON BJ. Chronic hepatitis B: Update 2009[J]. Hepatology, 2009, 50(3): 661-662. DOI: 10.1002/hep.23190. |
| [4] |
LIAW YF, KAO JH, PIRATVISUTH T, et al. Erratum to: Asian-Pacific consensus statement on the management of chronic hepatitis B: A 2012 update[J]. Hepatol Int, 2012, 6(4): 809-810. DOI: 10.1007/s12072-012-9386-z. |
| [5] |
LIAW YF, LAU GK, KAO JH, et al. Hepatitis B e antigen seroconversion: A critical event in chronic hepatitis B virus infection[J]. Dig Dis Sci, 2010, 55(10): 2727-2734. DOI: 10.1007/s10620-010-1179-4. |
| [6] |
FAN R, SUN J, YUAN Q, et al. Baseline quantitative hepatitis B core antibody titre alone strongly predicts HBeAg seroconversion across chronic hepatitis B patients treated with peginterferon or nucleos(t)ide analogues[J]. Gut, 2016, 65(2): 313-320. DOI: 10.1136/gutjnl-2014-308546. |
| [7] |
YUAN Q, SONG LW, LIU CJ, et al. Quantitative hepatitis B core antibody level may help predict treatment response in chronic hepatitis B patients[J]. Gut, 2013, 62(1): 182-184. DOI: 10.1136/gutjnl-2012-302656. |
| [8] |
CHI H, LI Z, HANSEN BE, et al. Serum level of antibodies against hepatitis B core protein is associated with clinical relapse after discontinuation of nucleos(t)ide analogue therapy[J]. Clin Gastroenterol Hepatol, 2019, 17(1): 182-191. e1. DOI: 10.1016/j.cgh.2018.05.047. |
| [9] |
WANG X, DONG Q, LI Q, et al. Dysregulated response of follicular helper t cells to hepatitis B surface antigen promotes HBV persistence in mice and associates with outcomes of patients[J]. Gastroenterology, 2018, 154(8): 2222-2236. DOI: 10.1053/j.gastro.2018.03.021. |
| [10] |
LIU XJ, ZHANG Z. Functional cure of chronic hepatitis B from the perspective of specific immune cells for hepatitis B virus[J]. J Clin Hepatol, 2020, 36(5): 977-979. DOI: 10.3969/j.issn.1001-5256.2020.05.004. |
| [11] |
SALIMZADEH L, LE BERT N, DUTERTRE CA, et al. PD-1 blockade partially recovers dysfunctional virus-specific B cells in chronic hepatitis B infection[J]. J Clin Invest, 2018, 128(10): 4573-4587. DOI: 10.1172/JCI121957. |
| [12] |
MILICH DR, MCLACHLAN A. The nucleocapsid of hepatitis B virus is both a T-cell-independent and a T-cell-dependent antigen[J]. Science, 1986, 234(4782): 1398-1401. DOI: 10.1126/science.3491425. |
| [13] |
CHU CM, LIAW YF. Intrahepatic distribution of hepatitis B surface and core antigens in chronic hepatitis B virus infection. Hepatocyte with cytoplasmic/membranous hepatitis B core antigen as a possible target for immune hepatocytolysis[J]. Gastroenterology, 1987, 92(1): 220-225. DOI: 10.1016/0016-5085(87)90863-8. |
| [14] |
CHU CM, LIAW YF. Immunohistological study of intrahepatic expression of hepatitis B core and e antigens in chronic type B hepatitis[J]. J Clin Pathol, 1992, 45(9): 791-795. DOI: 10.1136/jcp.45.9.791. |
| [15] |
SAITO T, KAMIMURA T, ISHIBASHI M, et al. Electron microscopic study of hepatitis B virus-associated antigens on the infected liver cell membrane in relation to analysis of immune target antigens in chronic hepatitis B[J]. Gastroenterol Jpn, 1992, 27(6): 734-744. DOI: 10.1007/BF02806526. |
| [16] |
CHEN HS, WU JF, SU TH, et al. Baseline level of hepatitis B core antibody predicts spontaneous hepatitis B e antigen (HBeAg) seroconversion in HBeAg-positive children with a normal alanine aminotransferase level[J]. Hepatology, 2019, 70(6): 1903-1912. DOI: 10.1002/hep.30788. |
| [17] |
VANWOLLEGHEM T, GROOTHUISMINK Z, KREEFFT K, et al. Hepatitis B core-specific memory B cell responses associate with clinical parameters in patients with chronic HBV[J]. J Hepatol, 2020, 73(1): 52-61. DOI: 10.1016/j.jhep.2020.01.024. |



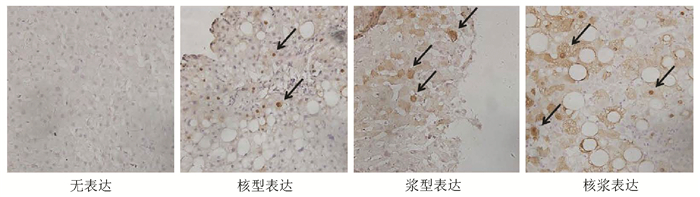




 DownLoad:
DownLoad: