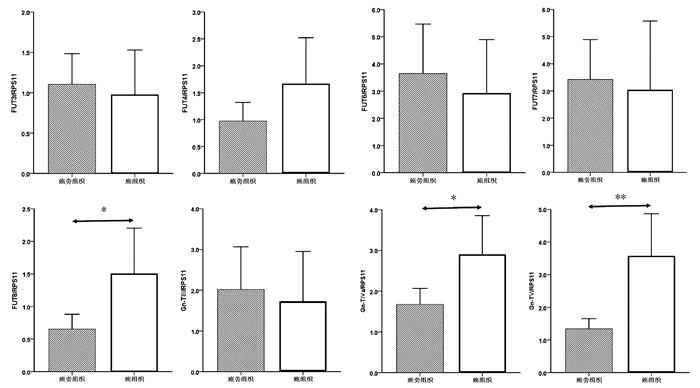
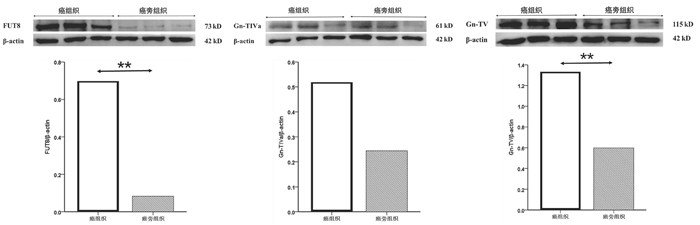
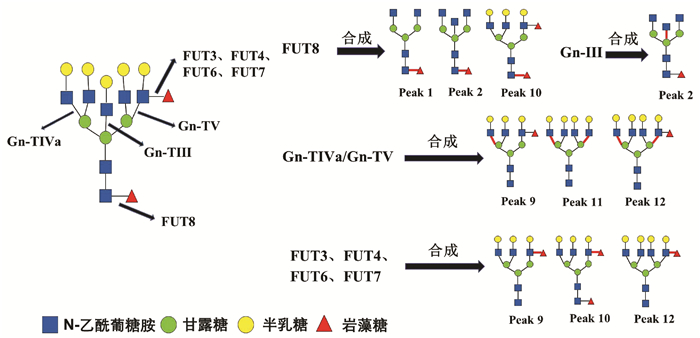
| [1] |
BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492. |
| [2] |
DRAKE RR. Glycosylation and cancer: Moving glycomics to the forefront[J]. Adv Cancer Res, 2015, 126: 1-10. DOI: 10.1016/bs.acr.2014.12.002. |
| [3] |
HÄUSELMANN I, BORSIG L. Altered tumor-cell glycosylation promotes metastasis[J]. Front Oncol, 2014, 4: 28. DOI: 10.3389/fonc.2014.00028. |
| [4] |
LIU XE, DESMYTER L, GAO CF, et al. N-glycomic changes in hepatocellular carcinoma patients with liver cirrhosis induced by hepatitis B virus[J]. Hepatology, 2007, 46(5): 1426-1435. DOI: 10.1002/hep.21855. |
| [5] |
Bureau of Medical Administration, National Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China(2019 edition)[J]. J Clin Hepatol, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007. |
| [6] |
CAO X, SHANG QH, CHI XL, et al. Serum N-glycan markers for diagnosing liver fibrosis induced by hepatitis B virus[J]. World J Gastroenterol, 2020, 26(10): 1067-1079. DOI: 10.3748/wjg.v26.i10.1067. |
| [7] |
CHENG L, GAO S, SONG X, et al. Comprehensive N-glycan profiles of hepatocellular carcinoma reveal association of fucosylation with tumor progression and regulation of FUT8 by microRNAs[J]. Oncotarget, 2016, 7(38): 61199-61214. DOI: 10.18632/oncotarget.11284. |
| [8] |
CHEN C, DIAO D, GUO L, et al. All-trans-retinoic acid modulates ICAM-1 N-glycan composition by influencing GnT-Ⅲ levels and inhibits cell adhesion and trans-endothelial migration[J]. PLoS One, 2012, 7(12): e52975. DOI: 10.1371/journal.pone.0052975. |
| [9] |
NIE H, LIU X, ZHANG Y, et al. Specific N-glycans of Hepatocellular Carcinoma Cell Surface and the Abnormal Increase of Core-α-1, 6-fucosylated Triantennary Glycan via N-acetylglucosaminyltransferases-IVa Regulation[J]. Sci Rep, 2015, 5: 16007. DOI: 10.1038/srep16007. |
| [10] |
WANG G, LIU J, CAI Y, et al. Loss of Barx1 promotes hepatocellular carcinoma metastasis through up-regulating MGAT5 and MMP9 expression and indicates poor prognosis[J]. Oncotarget, 2017, 8(42): 71867-71880. DOI: 10.18632/oncotarget.18288. |
| [11] |
WU X, DAO THI VL, LIU P, et al. Pan-genotype hepatitis E virus replication in stem cell-derived hepatocellular systems[J]. Gastroenterology, 2018, 154(3): 663-674. e7. DOI: 10.1053/j.gastro.2017.10.041. |
| [12] |
KIZUKA Y, TANIGUCHI N. Enzymes for N-glycan branching and their genetic and nongenetic regulation in cancer[J]. Biomolecules, 2016, 6(2). DOI: 10.3390/biom6020025. |
| [13] |
FANG M, ZHAO YP, ZHOU FG, et al. N-glycan based models improve diagnostic efficacies in hepatitis B virus-related hepatocellular carcinoma[J]. Int J Cancer, 2010, 127(1): 148-159. DOI: 10.1002/ijc.25030. |
| [14] |
CUI J, HUANG W, WU B, et al. N-glycosylation by N-acetylglucosaminyltransferase V enhances the interaction of CD147/basigin with integrin β1 and promotes HCC metastasis[J]. J Pathol, 2018, 245(1): 41-52. DOI: 10.1002/path.5054. |
| [15] |
FAN J, WANG S, YU S, et al. N-acetylglucosaminyltransferase IVa regulates metastatic potential of mouse hepatocarcinoma cells through glycosylation of CD147[J]. Glycoconj J, 2012, 29(5-6): 323-334. DOI: 10.1007/s10719-012-9414-1. |
| [16] |
ZHAO Y, NAKAGAWA T, ITOH S, et al. N-acetylglucosaminyltransferase Ⅲ antagonizes the effect of N-acetylglucosaminyltransferase V on alpha3beta1 integrin-mediated cell migration[J]. J Biol Chem, 2006, 281(43): 32122-32130. DOI: 10.1074/jbc.M607274200. |
| [17] |
ZHAO Y, ITOH S, WANG X, et al. Deletion of core fucosylation on alpha3beta1 integrin down-regulates its functions[J]. J Biol Chem, 2006, 281(50): 38343-38350. DOI: 10.1074/jbc.M608764200. |
| [18] |
DEBRUYNE EN, DELANGHE JR. Diagnosing and monitoring hepatocellular carcinoma with alpha-fetoprotein: New aspects and applications[J]. Clin Chim Acta, 2008, 395(1-2): 19-26. DOI: 10.1016/j.cca.2008.05.010. |
| [19] |
KEELEY TS, YANG S, LAU E. The diverse contributions of fucose linkages in cancer[J]. Cancers (Basel), 2019, 11(9). DOI: 10.3390/cancers11091241. |



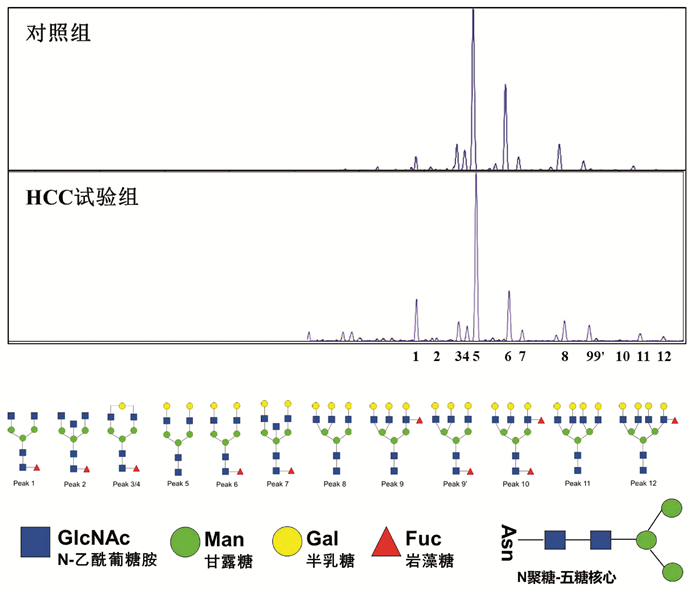




 DownLoad:
DownLoad: