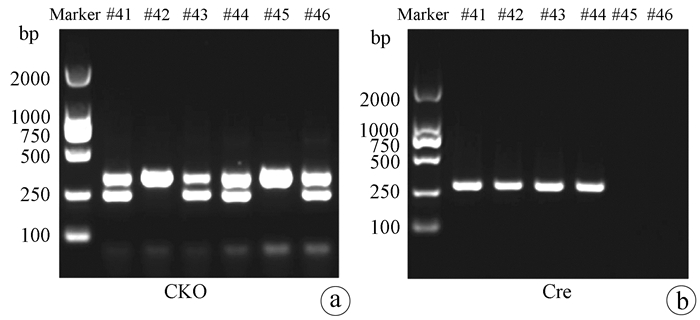
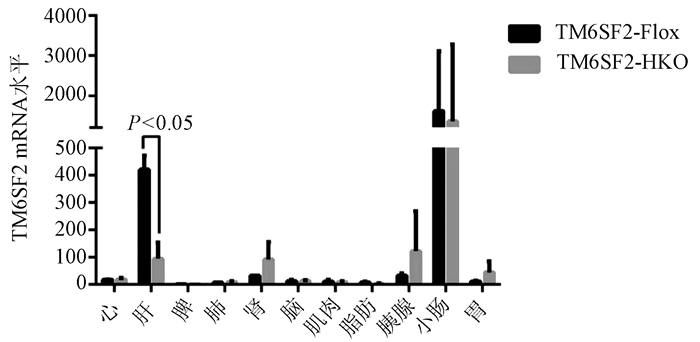
| [1] |
COBBINA E, AKHLAGHI F. Non-alcoholic fatty liver disease (NAFLD) - pathogenesis, classification, and effect on drug metabolizing enzymes and transporters[J]. Drug Metab Rev, 2017, 49(2): 197-211. DOI: 10.1080/03602532.2017.1293683. |
| [2] |
WU TF, LIAO XH, ZHONG BH. Epidemiology of nonalcoholic fatty liver disease in some regions of China[J]. J Clin Hepatol, 2020, 36(6): 1370-1373. DOI: 10.3969/j.issn.1001-5256.2020.06.039. |
| [3] |
YOUNOSSI Z, ANSTEE QM, MARIETTI M, et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention[J]. Nat Rev Gastroenterol Hepatol, 2018, 15(1): 11-20. DOI: 10.1038/nrgastro.2017.109. |
| [4] |
BYRNE CD, TARGHER G. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease: Is universal screening appropriate?[J]. Diabetologia, 2016, 59(6): 1141-1144. DOI: 10.1007/s00125-016-3910-y. |
| [5] |
LEUNG C, RIVERA L, FURNESS JB, et al. The role of the gut microbiota in NAFLD[J]. Nat Rev Gastroenterol Hepatol, 2016, 13(7): 412-425. DOI: 10.1038/nrgastro.2016.85. |
| [6] |
KOZLITINA J, SMAGRIS E, STENDER S, et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease[J]. Nat Genet, 2014, 46(4): 352-356. DOI: 10.1038/ng.2901. |
| [7] |
SOOKOIAN S, CASTAÑO GO, SCIAN R, et al. Genetic variation in transmembrane 6 superfamily member 2 and the risk of nonalcoholic fatty liver disease and histological disease severity[J]. Hepatology, 2015, 61(2): 515-525. DOI: 10.1002/hep.27556. |
| [8] |
KRAWCZYK M, RAU M, SCHATTENBERG JM, et al. Combined effects of the PNPLA3 rs738409, TM6SF2 rs58542926, and MBOAT7 rs641738 variants on NAFLD severity: A multicenter biopsy-based study[J]. J Lipid Res, 2017, 58(1): 247-255. DOI: 10.1194/jlr.P067454. |
| [9] |
GOFFREDO M, CAPRIO S, FELDSTEIN AE, et al. Role of TM6SF2 rs58542926 in the pathogenesis of nonalcoholic pediatric fatty liver disease: A multiethnic study[J]. Hepatology, 2016, 63(1): 117-125. DOI: 10.1002/hep.28283. |
| [10] |
ANDRIKOPOULOS S, BLAIR AR, DELUCA N, et al. Evaluating the glucose tolerance test in mice[J]. Am J Physiol Endocrinol Metab, 2008, 295(6): E1323-1332. DOI: 10.1152/ajpendo.90617.2008. |
| [11] |
MA Y, YU L, PAN S, et al. CRISPR/Cas9-mediated targeting of the Rosa26 locus produces Cre reporter rat strains for monitoring Cre-loxP-mediated lineage tracing[J]. FEBS J, 2017, 284(19): 3262-3277. DOI: 10.1111/febs.14188. |
| [12] |
FAN Y, LU H, GUO Y, et al. Hepatic Transmembrane 6 superfamily member 2 regulates cholesterol metabolism in mice[J]. Gastroenterology, 2016, 150(5): 1208-1218. DOI: 10.1053/j.gastro.2016.01.005. |
| [13] |
GUPTA D, BHATTACHARJEE O, MANDAL D, et al. CRISPR-Cas9 system: A new-fangled dawn in gene editing[J]. Life Sci, 2019, 232: 116636. DOI: 10.1016/j.lfs.2019.116636. |
| [14] |
HRYHOROWICZ M, LIPIŃSKI D, ZEYLAND J, et al. CRISPR/Cas9 immune system as a tool for genome engineering[J]. Arch Immunol Ther Exp (Warsz), 2017, 65(3): 233-240. DOI: 10.1007/s00005-016-0427-5. |
| [15] |
YANG J, ZHU D, JU B, et al. Hepatoprotective effects of Gentianella turkestanerum extracts on acute liver injury induced by carbon tetrachloride in mice[J]. Am J Transl Res, 2017, 9(2): 569-579.
|
| [16] |
HWANG YP, CHOI JH, JEONG HG. Protective effect of the Aralia continentalis root extract against carbon tetrachloride-induced hepatotoxicity in mice[J]. Food Chem Toxicol, 2009, 47(1): 75-81. DOI: 10.1016/j.fct.2008.10.011. |
| [17] |
Diagnosis and Treatment Center of Hepatology of South China Alliance of TCM, National Administration of Traditional Chinese Medicine. Diagnosis and treatment scheme of Ganpi (non-alcoholic steatohepatitis)[J/CD]. Chin J Liver Dis: Electronic Edition, 2021, 13(1): 1-9. DOI: 10.3969/j.issn.1674-7380.2021.01.001 |
| [18] |
PETERSEN MC, VATNER DF, SHULMAN GI. Regulation of hepatic glucose metabolism in health and disease[J]. Nat Rev Endocrinol, 2017, 13(10): 572-587. DOI: 10.1038/nrendo.2017.80. |
| [19] |
WATT MJ, MIOTTO PM, de NARDO W, et al. The liver as an endocrine organ-linking nafld and insulin resistance[J]. Endocr Rev, 2019, 40(5): 1367-1393. DOI: 10.1210/er.2019-00034. |



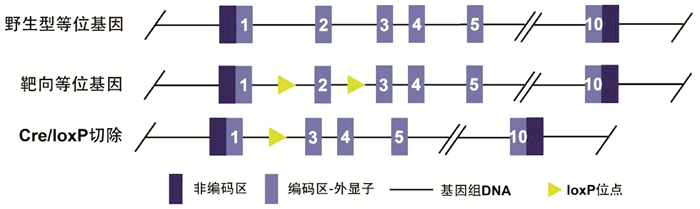




 DownLoad:
DownLoad: