| [1] |
|
| [2] |
European Association for the Study of the Liver. EASL clinical practice guidelines: Management of hepatocellular carcinoma[J]. J Hepatol, 2018, 69(1): 182-236. DOI: 10.1016/j.jhep.2018.03.019. |
| [3] |
ELLEGAARD O, WALLIN JA. The bibliometric analysis of scholarly production: How great is the impact?[J]. Scientometrics, 2015, 105(3): 1809-1831. DOI: 10.1007/s11192-015-1645-z. |
| [4] |
YANG HY, WANG D, CHEN C, et al. Global research status of gastroenterology and hepatology: A bibliometrics study[J]. Medicine (Baltimore), 2021, 100(15): e25291. DOI: 10.1097/MD.0000000000025291. |
| [5] |
HU SK, HUANG J, HONG WD, et al. The 50 most-cited articles in gastroenterology and hepatology from Mainland China[J]. Pak J Med Sci, 2017, 33(1): 215-220. DOI: 10.12669/pjms.331.12286. |
| [6] |
|
| [7] |
LI G, LIN J, JIANG C, et al. Trends in chronic hepatitis B treatment-related research from 1973 to 2018: A bibliometric and visual analysis[J]. J Int Med Res, 2020, 48(4): 300060519893234. DOI: 10.1177/0300060519893234. |
| [8] |
XU G, JIN B, XIAN X, et al. Evolutions in the management of hepatocellular carcinoma over last 4 decades: An analysis from the 100 most influential articles in the field[J]. Liver Cancer, 2021, 10(2): 137-150. DOI: 10.1159/000513412. |
| [9] |
HU AGZ. Public funding and the ascent of Chinese science: Evidence from the National Natural Science Foundation of China[J]. Research Policy, 2020, 49(5): 13. DOI: 10.1016/j.respol.2020.103983. |
| [10] |
LIU Y, GAO Z, WANG H, et al. Analysis of projects funded by the National Natural Science Foundation of China during the years of 2014-2018[J]. Ann Transl Med, 2019, 7(12): 267. DOI: 10.21037/atm.2019.05.63. |
| [11] |
|
| [12] |
ARIA M, CUCCURULLO C. bibliometrix: An R-tool for comprehensive science mapping analysis[J]. J Informetr, 2017, 11(4): 959-975. DOI: 10.1016/j.joi.2017.08.007. |
| [13] |
|
| [14] |
NESHAT SY, QUIROZ VM, WANG Y, et al. Liver disease: Induction, progression, immunological mechanisms, and therapeutic interventions[J]. Int J Mol Sci, 2021, 22(13): 6777. DOI: 10.3390/ijms22136777. |
| [15] |
IOANNOU GN, GREEN PK, BERRY K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma[J]. J Hepatol, 2018, 68(1): 25-32. DOI: 10.1016/j.jhep.2017.08.030. |
| [16] |
EL-KHOUEIRY AB, SANGRO B, YAU T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial[J]. Lancet, 2017, 389(10088): 2492-2502. DOI: 10.1016/S0140-6736(17)31046-2. |
| [17] |
BRUIX J, QIN S, MERLE P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial[J]. Lancet, 2017, 389(10064): 56-66. DOI: 10.1016/S0140-6736(16)32453-9. |
| [18] |
LOOMBA R, ADAMS LA. Advances in non-invasive assessment of hepatic fibrosis[J]. Gut, 2020, 69(7): 1343-1352. DOI: 10.1136/gutjnl-2018-317593. |
| [19] |
RUDIGER MS, ANTONS D, SALGE TO. The explanatory power of citations: A new approach to unpacking impact in science[J]. Scientometrics, 2021, 126: 9779-9809. DOI: 10.1007/s11192-021-04103-w. |
| [20] |
ZHU HR. Home country bias in academic publishing: A case study of the New England Journal of Medicine[J]. Learned Publishing, 2021, 34(4): 578-584. DOI: 10.1002/leap.1404. |
| [21] |
WANG J, SHEN T, HUANG X, et al. Serum hepatitis B virus RNA is encapsidated pregenome RNA that may be associated with persistence of viral infection and rebound[J]. J Hepatol, 2016, 65(4): 700-710. DOI: 10.1016/j.jhep.2016.05.029. |
| [22] |
XU X, LU D, LING Q, et al. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria[J]. Gut, 2016, 65(6): 1035-1041. DOI: 10.1136/gutjnl-2014-308513. |
| [23] |
SUN Y, ZHOU J, WANG L, et al. New classification of liver biopsy assessment for fibrosis in chronic hepatitis B patients before and after treatment[J]. Hepatology, 2017, 65(5): 1438-1450. DOI: 10.1002/hep.29009. |
| [24] |
LV Y, QI X, HE C, et al. Covered TIPS versus endoscopic band ligation plus propranolol for the prevention of variceal rebleeding in cirrhotic patients with portal vein thrombosis: A randomised controlled trial[J]. Gut, 2018, 67(12): 2156-2168. DOI: 10.1136/gutjnl-2017-314634. |



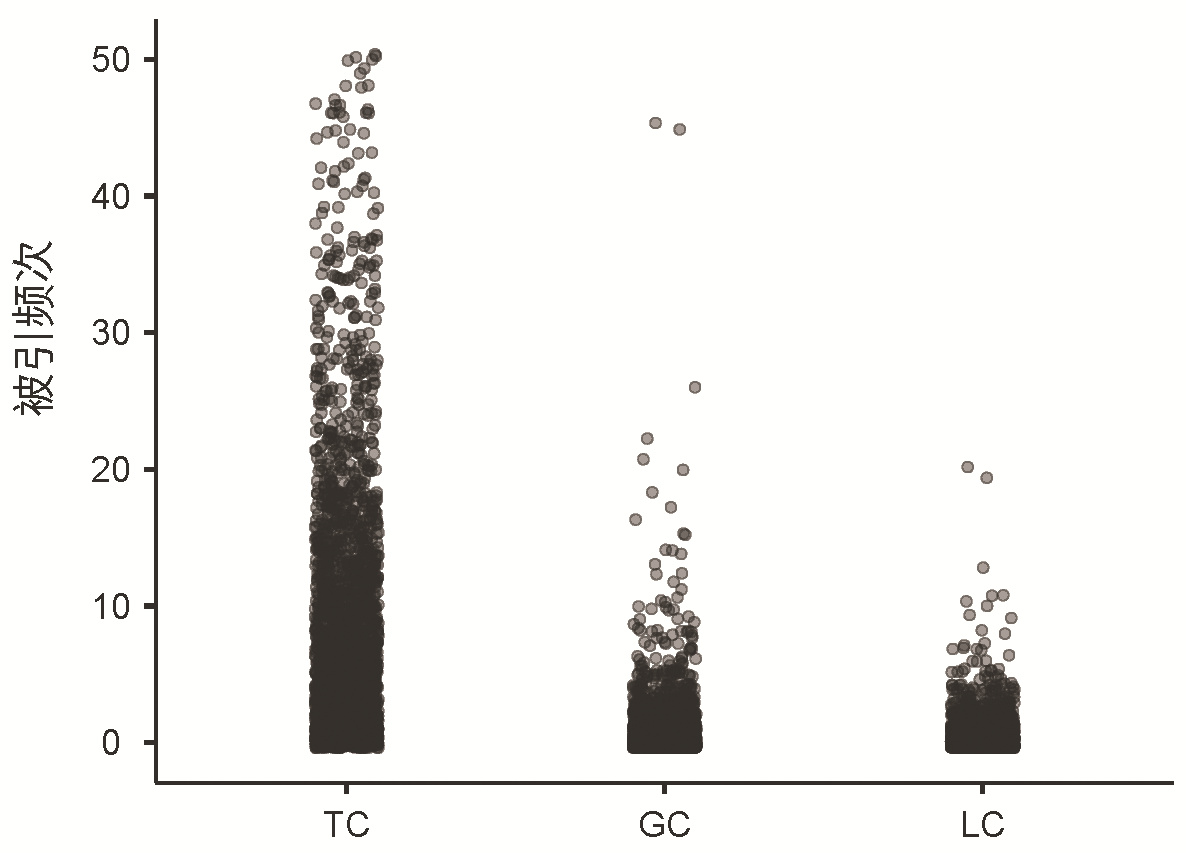




 DownLoad:
DownLoad: