| [1] |
RAMOS FIGUEIRA ER, ROCHA FILHO JA, SOUTO NACIF L, et al. Nutritional support for fulminant hepatitis[J]. Nutr Hosp, 2015, 32(6): 2427-2432. DOI: 10.3305/nh.2015.32.6.9769. |
| [2] |
LEE SM, BOOE JM, PIOSZAK AA. Structural insights into ligand recognition and selectivity for classes A, B, and C GPCRs[J]. Eur J Pharmacol, 2015, 763(Pt B): 196-205. DOI: 10.1016/j.ejphar.2015.05.013. |
| [3] |
SCHÖNEBERG T, LIEBSCHER I. Mutations in G protein-coupled receptors: mechanisms, pathophysiology and potential therapeutic approaches[J]. Pharmacol Rev, 2021, 73(1): 89-119. DOI: 10.1124/pharmrev.120.000011. |
| [4] |
PENG WT, SUN WY, LI XR, et al. Emerging roles of G protein-coupled receptors in hepatocellular carcinoma[J]. Int J Mol Sci, 2018, 19(5): 1366. DOI: 10.3390/ijms19051366. |
| [5] |
DUC NM, KIM HR, CHUNG KY. Structural mechanism of G protein activation by G protein-coupled receptor[J]. Eur J Pharmacol, 2015, 763(Pt B): 214-222. DOI: 10.1016/j.ejphar.2015.05.016. |
| [6] |
HAUSER AS, ATTWOOD MM, RASK-ANDERSEN M, et al. Trends in GPCR drug discovery: new agents, targets and indications[J]. Nat Rev Drug Discov, 2017, 16(12): 829-842. DOI: 10.1038/nrd.2017.178. |
| [7] |
GOUGH NR. Focus issue: New insights in GPCR to G protein signaling[J]. Sci Signal, 2016, 9(423): eg6. DOI: 10.1126/scisignal.aaf7642. |
| [8] |
CATTANEO F, GUERRA G, PARISI M, et al. Cell-surface receptors transactivation mediated by g protein-coupled receptors[J]. Int J Mol Sci, 2014, 15(11): 19700-19728. DOI: 10.3390/ijms151119700. |
| [9] |
SCHÖNEBERG T, SCHULZ A, BIEBERMANN H, et al. Mutant G-protein-coupled receptors as a cause of human diseases[J]. Pharmacol Ther, 2004, 104(3): 173-206. DOI: 10.1016/j.pharmthera.2004.08.008. |
| [10] |
ZHOU J, ZHOU F, WANG W, et al. Epidemiological features of NAFLD from 1999 to 2018 in China[J]. Hepatology, 2020, 71(5): 1851-1864. DOI: 10.1002/hep.31150. |
| [11] |
REVIEW TEAM, LABRECQUE DR, ABBAS Z, et al. World Gastroenterology Organisation global guidelines: Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis[J]. J Clin Gastroenterol, 2014, 48(6): 467-473. DOI: 10.1097/MCG.0000000000000116. |
| [12] |
FARRELL GC, van ROOYEN D, GAN L, et al. NASH is an inflammatory disorder: pathogenic, prognostic and therapeutic implications[J]. Gut Liver, 2012, 6(2): 149-171. DOI: 10.5009/gnl.2012.6.2.149. |
| [13] |
BAHIRAT UA, SHENOY RR, TALWAR R, et al. Co-administration of APD668, a G protein-coupled receptor 119 agonist and linagliptin, a DPPIV inhibitor, prevents progression of steatohepatitis in mice fed on a high trans-fat diet[J]. Biochem Biophys Res Commun, 2018, 495(2): 1608-1613. DOI: 10.1016/j.bbrc.2017.12.004. |
| [14] |
YANG JW, KIM HS, IM JH, et al. GPR119: a promising target for nonalcoholic fatty liver disease[J]. FASEB J, 2016, 30(1): 324-335. DOI: 10.1096/fj.15-273771. |
| [15] |
CHEN X, LIU C, RUAN L. G-protein-coupled receptors 120 agonist iii improves hepatic inflammation and ER stress in steatohepatitis[J]. Dig Dis Sci, 2021, 66(4): 1090-1096. DOI: 10.1007/s10620-020-06280-9. |
| [16] |
AYD 1N MM, AKÇAL 1 KC. Liver fibrosis[J]. Turk J Gastroenterol, 2018, 29(1): 14-21. DOI: 10.5152/tjg.2018.17330. |
| [17] |
Chinese Society of Hepatology, Chinese Medical Association. Chinese guidelines on the management of liver cirrhosis[J]. J Clin Hepatol, 2019, 35(11): 2408-2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006. |
| [18] |
LOTERSZTAJN S, TEIXEIRA-CLERC F, JULIEN B, et al. CB2 receptors as new therapeutic targets for liver diseases[J]. Br J Pharmacol, 2008, 153(2): 286-289. DOI: 10.1038/sj.bjp.0707511. |
| [19] |
JULIEN B, GRENARD P, TEIXEIRA-CLERC F, et al. Antifibrogenic role of the cannabinoid receptor CB2 in the liver[J]. Gastroenterology, 2005, 128(3): 742-755. DOI: 10.1053/j.gastro.2004.12.050. |
| [20] |
de CASTRO FONSECA M, AGUIAR CJ, da ROCHA FRANCO JA, et al. GPR91: expanding the frontiers of Krebs cycle intermediates[J]. Cell Commun Signal, 2016, 14: 3. DOI: 10.1186/s12964-016-0126-1. |
| [21] |
LIU XJ, XIE L, DU K, et al. Succinate-GPR-91 receptor signalling is responsible for nonalcoholic steatohepatitis-associated fibrosis: Effects of DHA supplementation[J]. Liver Int, 2020, 40(4): 830-843. DOI: 10.1111/liv.14370. |
| [22] |
NGUYEN G, PARK SY, LE CT, et al. Metformin ameliorates activation of hepatic stellate cells and hepatic fibrosis by succinate and GPR91 inhibition[J]. Biochem Biophys Res Commun, 2018, 495(4): 2649-2656. DOI: 10.1016/j.bbrc.2017.12.143. |
| [23] |
LI YH, WOO SH, CHOI DH, et al. Succinate causes α-SMA production through GPR91 activation in hepatic stellate cells[J]. Biochem Biophys Res Commun, 2015, 463(4): 853-858. DOI: 10.1016/j.bbrc.2015.06.023. |
| [24] |
PARK SY, LE CT, SUNG KY, et al. Succinate induces hepatic fibrogenesis by promoting activation, proliferation, and migration, and inhibiting apoptosis of hepatic stellate cells[J]. Biochem Biophys Res Commun, 2018, 496(2): 673-678. DOI: 10.1016/j.bbrc.2018.01.106. |
| [25] |
LI Y, XU A, JIA S, et al. Recent advances in the molecular mechanism of sex disparity in hepatocellular carcinoma[J]. Oncol Lett, 2019, 17(5): 4222-4228. DOI: 10.3892/ol.2019.10127. |
| [26] |
YAN SY, FAN JG. Diagnosis and treatment of hepatocellular carcinoma associated with nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2021, 37(8): 1748-1752. DOI: 10.3969/j.issn.1001-5256.2021.08.002. |
| [27] |
CASTAN L, MAGNAN A, BOUCHAUD G. Chemokine receptors in allergic diseases[J]. Allergy, 2017, 72(5): 682-690. DOI: 10.1111/all.13089. |
| [28] |
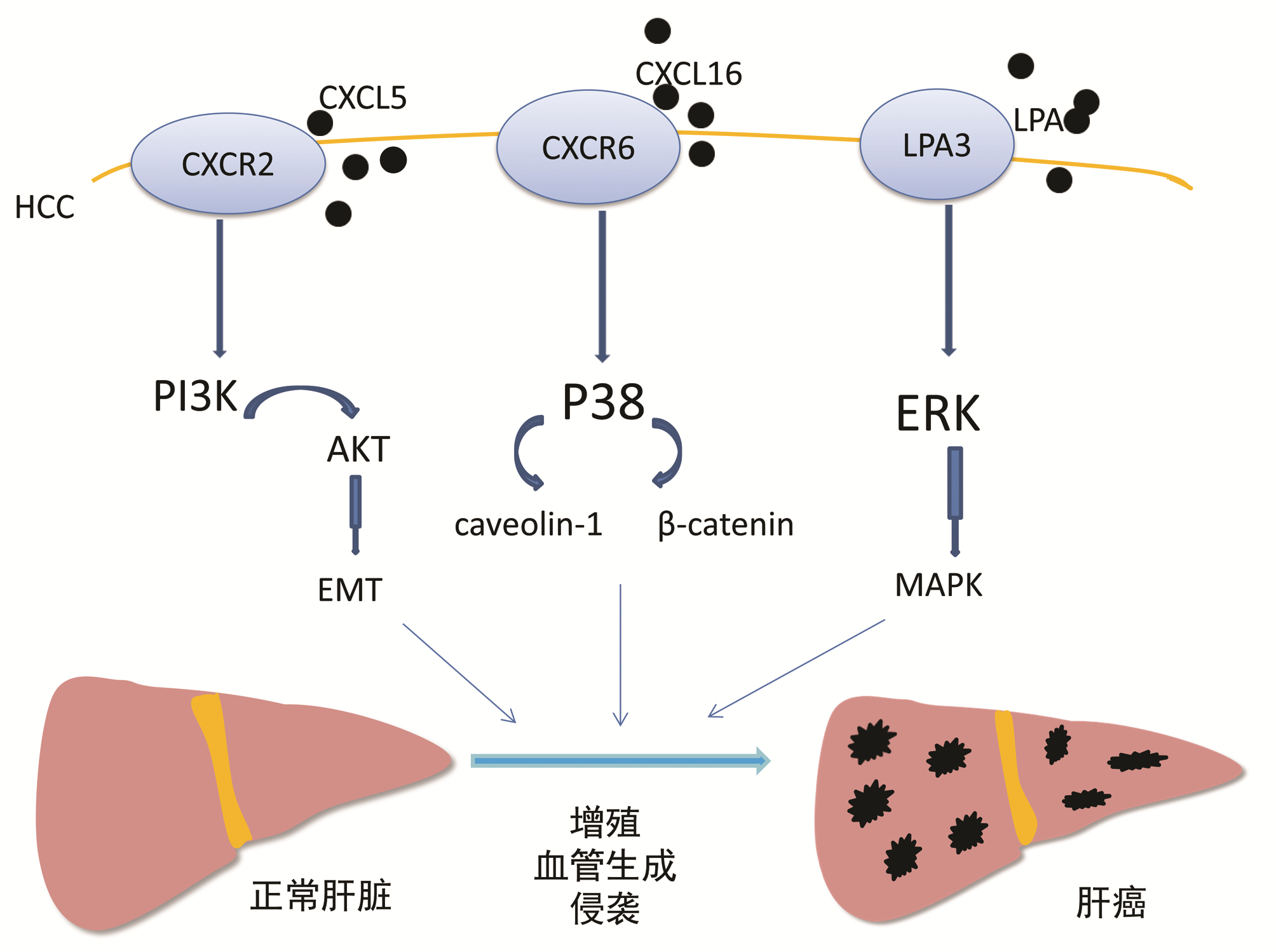
ZHOU SL, DAI Z, ZHOU ZJ, et al. Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma[J]. Hepatology, 2012, 56(6): 2242-2254. DOI: 10.1002/hep.25907. |
| [29] |
LI Y, WU J, ZHANG W, et al. Identification of serum CCL15 in hepatocellular carcinoma[J]. Br J Cancer, 2013, 108(1): 99-106. DOI: 10.1038/bjc.2012.494. |
| [30] |
LIU Z, YANG L, XU J, et al. Enhanced expression and clinical significance of chemokine receptor CXCR2 in hepatocellular carcinoma[J]. J Surg Res, 2011, 166(2): 241-246. DOI: 10.1016/j.jss.2009.07.014. |
| [31] |
ZHOU SL, ZHOU ZJ, HU ZQ, et al. CXCR2/CXCL5 axis contributes to epithelial-mesenchymal transition of HCC cells through activating PI3K/Akt/GSK-3β/Snail signaling[J]. Cancer Lett, 2015, 358(2): 124-135. DOI: 10.1016/j.canlet.2014.11.044. |
| [32] |
GAO Q, ZHAO YJ, WANG XY, et al. CXCR6 upregulation contributes to a proinflammatory tumor microenvironment that drives metastasis and poor patient outcomes in hepatocellular carcinoma[J]. Cancer Res, 2012, 72(14): 3546-3556. DOI: 10.1158/0008-5472.CAN-11-4032. |
| [33] |
YANAGIDA K, ISHⅡ S. Non-Edg family LPA receptors: the cutting edge of LPA research[J]. J Biochem, 2011, 150(3): 223-232. DOI: 10.1093/jb/mvr087. |
| [34] |
ZUCKERMAN V, SOKOLOV E, SWET JH, et al. Expression and function of lysophosphatidic acid receptors (LPARs) 1 and 3 in human hepatic cancer progenitor cells[J]. Oncotarget, 2016, 7(3): 2951-2967. DOI: 10.18632/oncotarget.6696. |
| [35] |
SOKOLOV E, EHEIM AL, AHRENS WA, et al. Lysophosphatidic acid receptor expression and function in human hepatocellular carcinoma[J]. J Surg Res, 2013, 180(1): 104-113. DOI: 10.1016/j.jss.2012.10.054. |







 DownLoad:
DownLoad: