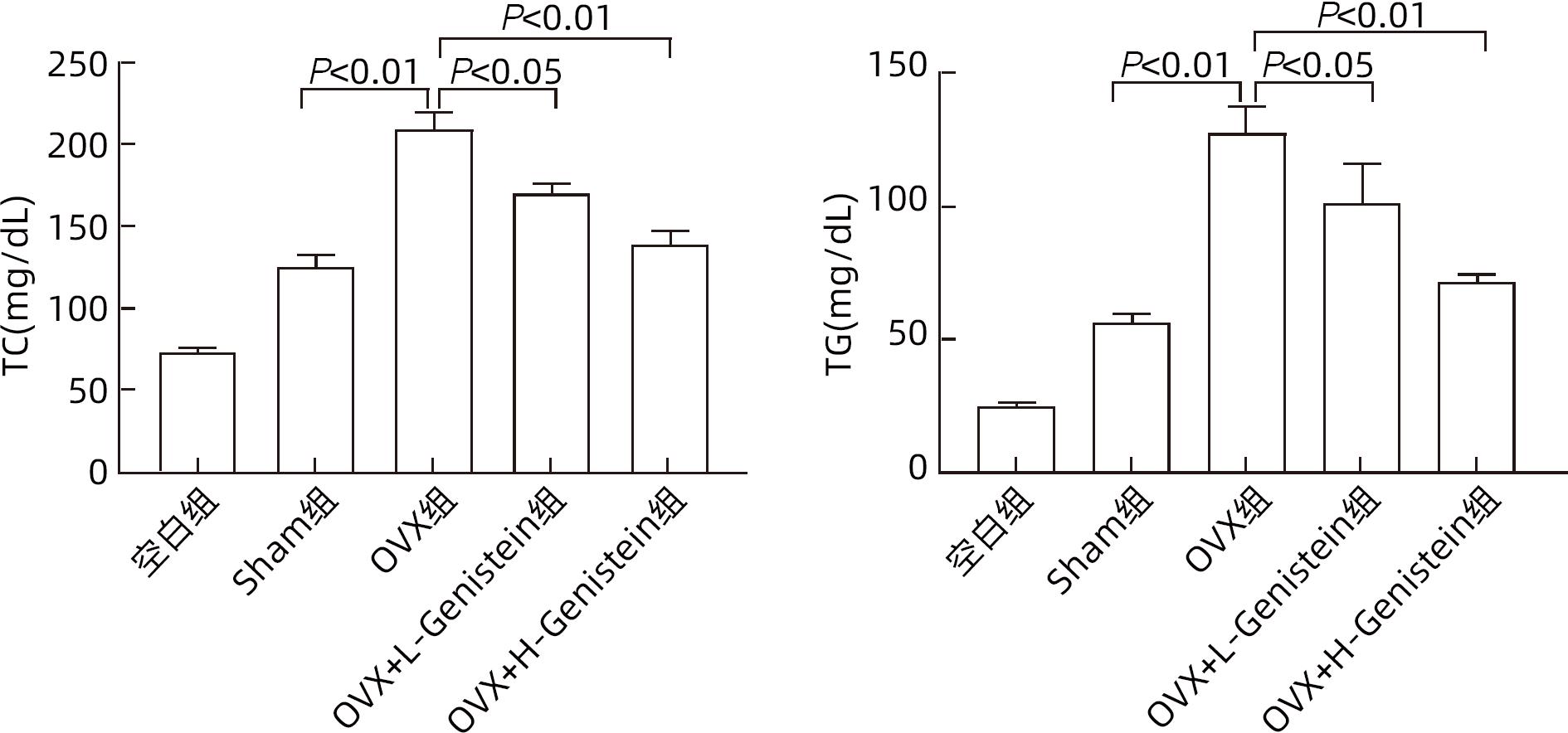
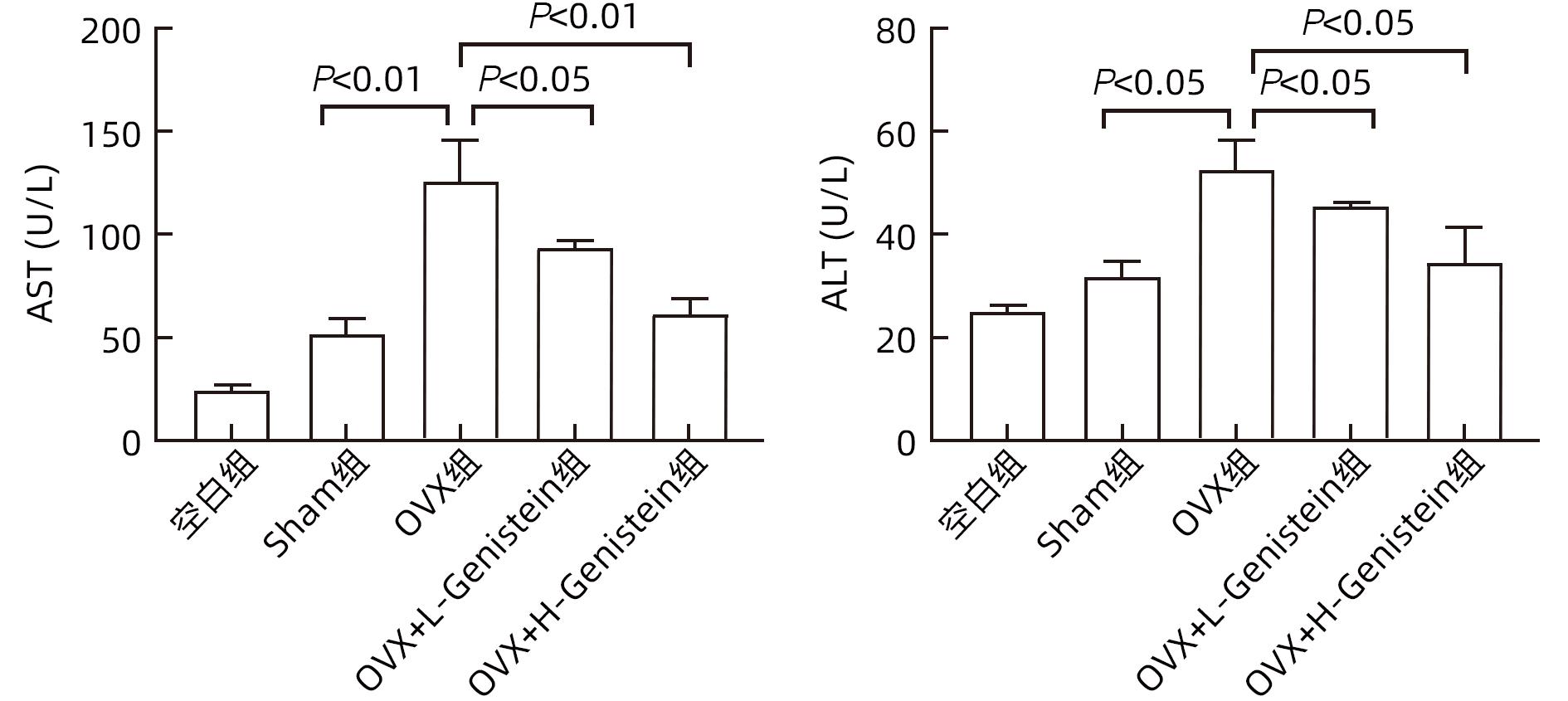
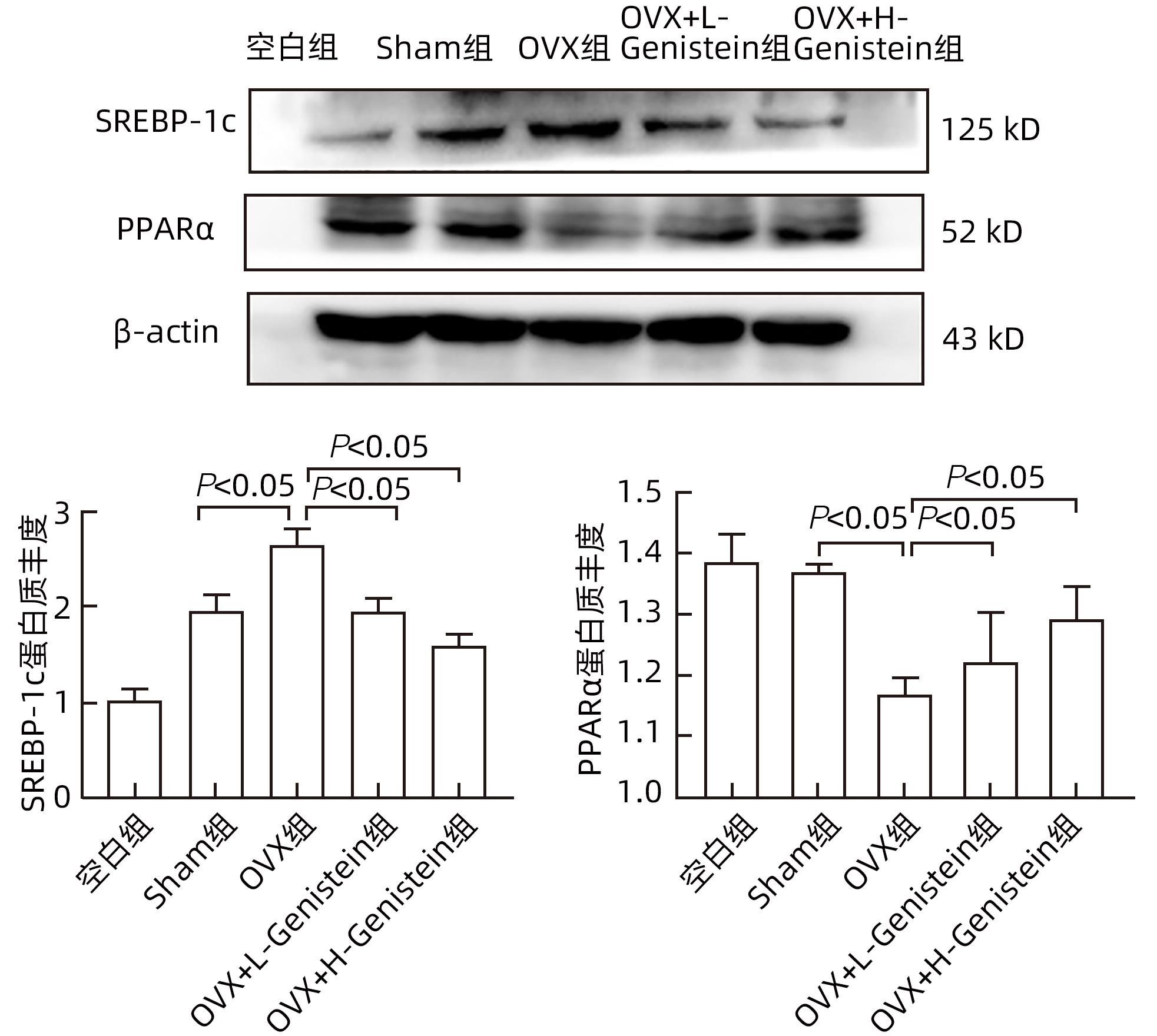
| [1] |
PAFILI K, RODEN M. Nonalcoholic fatty liver disease(NAFLD) from pathogenesis to treatment concepts in humans[J]. Mol Metab, 2021, 50: 101122. DOI: 10.1016/j.molmet.2020.101122. |
| [2] |
YU Y, CAI JJ, SHE ZG, et al. Insights into the epidemiology, pathogenesis, and therapeutics of nonalcoholic fatty liver diseases[J]. Adv Sci(Weinh), 2018, 6( 4): 1801585. DOI: 10.1002/advs.201801585. |
| [3] |
MANNE V, HANDA P, KOWDLEY KV. Current treatment of non-alcoholic fatty liver disease[J]. Clin Liver Dis, 2018, 22( 1): 23- 37. DOI: 10.1016/j.cld.2017.08.007. |
| [4] |
CHOUDHURI G, SHAH S, KULKARNI A, et al. Non-alcoholic steatohepatitis in asians: Current perspectives and future directions[J]. Cureus, 2023, 15( 8): e42852. DOI: 10.7759/cureus.42852. |
| [5] |
PAWLOWSKI JW, MARTIN BR, MCCABE GP, et al. Impact of equol-producing capacity and soy-isoflavone profiles of supplements on bone calcium retention in postmenopausal women: A randomized crossover trial[J]. Am J Clin Nutr, 2015, 102( 3): 695- 703. DOI: 10.3945/ajcn.114.093906. |
| [6] |
ZHAO L, WANG Y, LIU J, et al. Protective effects of genistein and puerarin against chronic alcohol-induced liver injury in mice via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms[J]. J Agric Food Chem, 2016, 64( 38): 7291- 7297. DOI: 10.1021/acs.jafc.6b02907. |
| [7] |
AMANAT S, EFTEKHARI MH, FARAROUEI M, et al. Genistein supplementation improves insulin resistance and inflammatory state in non-alcoholic fatty liver patients: A randomized, controlled trial[J]. Clin Nutr, 2018, 37( 4): 1210- 1215. DOI: 10.1016/j.clnu.2017.05.028. |
| [8] |
XIA HG, ZHU XY, ZHANG XY, et al. Alpha-naphthoflavone attenuates non-alcoholic fatty liver disease in oleic acid-treated HepG2 hepatocytes and in high fat diet-fed mice[J]. Biomed Pharmacother, 2019, 118: 109287. DOI: 10.1016/j.biopha.2019.109287. |
| [9] |
MOLINA-MOLINA E, FURTADO GE, JONES JG, et al. The advantages of physical exercise as a preventive strategy against NAFLD in postmenopausal women[J]. Eur J Clin Invest, 2022, 52( 3): e13731. DOI: 10.1111/eci.13731. |
| [10] |
ZHU XY, XIA HG, WANG ZH, et al. In vitro and in vivo approaches for identifying the role of aryl hydrocarbon receptor in the development of nonalcoholic fatty liver disease[J]. Toxicol Lett, 2020, 319: 85- 94. DOI: 10.1016/j.toxlet.2019.10.010. |
| [11] |
CALIGIONI CS. Assessing reproductive status/stages in mice[J]. Curr Protoc Neurosci, 2009, Appendix 4: Appendix 4 I. DOI: 10.1002/0471142301.nsa04is48. |
| [12] |
DEMIR M, BORNSTEIN SR, MANTZOROS CS, et al. Liver fat as risk factor of hepatic and cardiometabolic diseases[J]. Obes Rev, 2023, 24( 10): e13612. DOI: 10.1111/obr.13612. |
| [13] |
|
| [14] |
WANG CE, XU WT, GONG J, et al. Research progress in the treatment of non-alcoholic fatty liver disease[J]. Clin J Med Offic, 2022, 50( 9): 897- 899, 903. DOI: 10.16680/j.1671-3826.2022.09.06. |
| [15] |
FRIEDMAN SL, NEUSCHWANDER-TETRI BA, RINELLA M, et al. Mechanisms of NAFLD development and therapeutic strategies[J]. Nat Med, 2018, 24( 7): 908- 922. DOI: 10.1038/s41591-018-0104-9. |
| [16] |
ALEXANDER KS, ZAKAI NA, LIDOFSKY SD, et al. Non-alcoholic fatty liver disease, liver biomarkers and stroke risk: The reasons for geographic and racial differences in stroke cohort[J]. PLoS One, 2018, 13( 3): e0194153. DOI: 10.1371/journal.pone.0194153. |
| [17] |
KAWAMURA S, MATSUSHITA Y, KUROSAKI S, et al. Inhibiting SCAP/SREBP exacerbates liver injury and carcinogenesis in murine nonalcoholic steatohepatitis[J]. J Clin Invest, 2022, 132( 11): e151895. DOI: 10.1172/JCI151895. |








 DownLoad:
DownLoad: