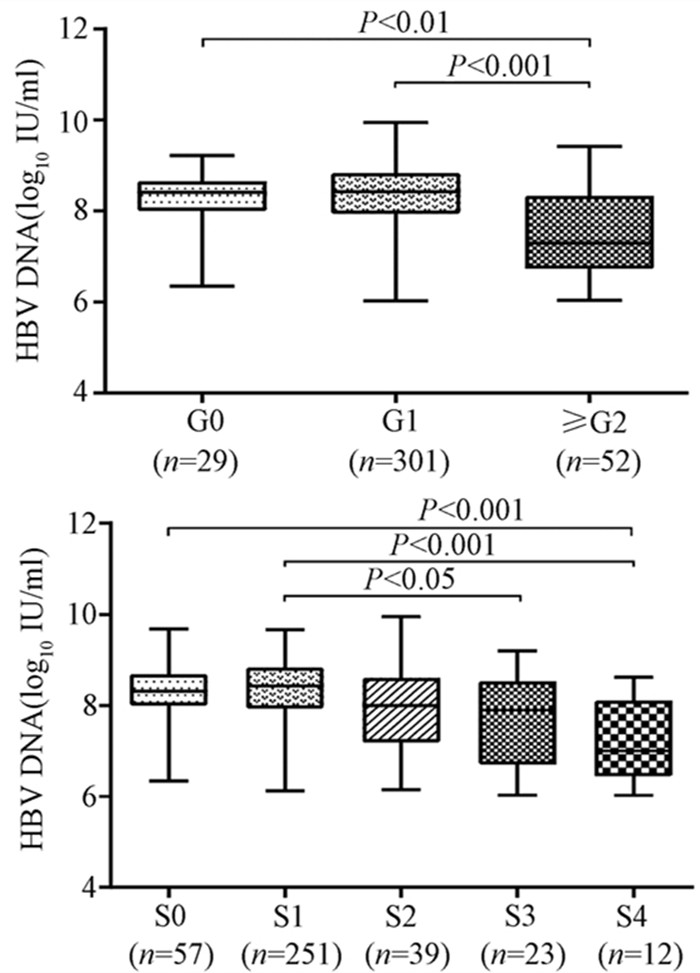
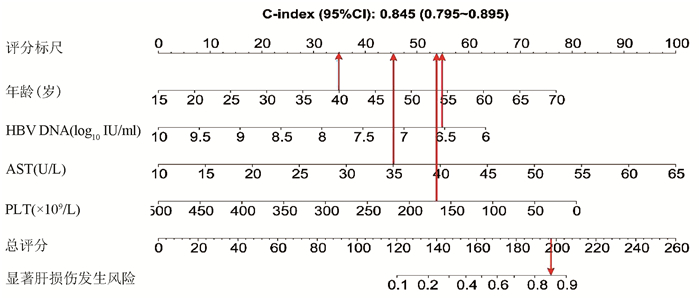
| [1] |
Chinese Society of Infectious Diseases, Chinese Medical Association, Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. |
| [2] |
TERRAULT NA, LOK A, MCMAHON BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance[J]. Hepatology, 2018, 67(4): 1560-1599. DOI: 10.1002/hep.29800. |
| [3] |
SARIN SK, KUMAR M, LAU GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update[J]. Hepatol Int, 2016, 10(1): 1-98. DOI: 10.1007/s12072-015-9675-4. |
| [4] |
European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection[J]. J Hepatol, 2017, 67(2): 370-398. DOI: 10.1016/j.jhep.2017.03.021. |
| [5] |
GAO HY, LIU N, LI CX, et al. Clinical features and liver histopathological analysis of patients in the immune tolerance stage of chronic hepatitis B virus infection[J]. Chin Hepatol, 2018, 23(2): 136-139. DOI: 10.14000/j.cnki.issn.1008-1704.2018.02.012. |
| [6] |
XING YF, ZHOU DQ, HE JS, et al. Clinical and histopathological features of chronic hepatitis B virus infected patients with high HBV DNA viral load and normal alanine aminotransferase level: A multicentre-based study in China[J]. PLoS One, 2018, 13(9): e0203220. DOI: 10.1371/journal.pone.0203220. |
| [7] |
KUMAR M, SARIN SK, HISSAR S, et al. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT[J]. Gastroenterology, 2008, 134(5): 1376-1384. DOI: 10.1053/j.gastro.2008.02.075. |
| [8] |
NGUYEN MH, GARCIA RT, TRINH HN, et al. Histological disease in Asian-Americans with chronic hepatitis B, high hepatitis B virus DNA, and normal alanine aminotransferase levels[J]. Am J Gastroenterol, 2009, 104(9): 2206-2213. DOI: 10.1038/ajg.2009.248. |
| [9] |
SETO WK, LAI CL, IP PP, et al. A large population histology study showing the lack of association between ALT elevation and significant fibrosis in chronic hepatitis B[J]. PLoS One, 2012, 7(2): e32622. DOI: 10.1371/journal.pone.0032622. |
| [10] |
KIM HL, KIM GA, PARK JA, et al. Cost-effectiveness of antiviral treatment in adult patients with immune-tolerant phase chronic hepatitis B[J]. Gut, 2020. [Online ahead of print]. DOI: 10.1136/gutjnl-2020-321309. |
| [11] |
KIM GA, LIM YS, HAN S, et al. High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis B[J]. Gut, 2018, 67(5): 945-952. DOI: 10.1136/gutjnl-2017-314904. |
| [12] |
GAO HY, LIU N, LI CX, et al. Research advances in influencing factors for natural prognosis of patients with chronic HBV infection in immune tolerance phase[J]. J Clin Hepatol, 2017, 33(8): 1572-1575. DOI: 10.3969/j.issn.1001-5256.2017.08.035. 高红艳, 刘娜, 李春霞, 等. 慢性HBV感染者免疫耐受期自然转归相关影响因素的研究进展[J]. 临床肝胆病杂志, 2017, 33(8): 1572-1575. DOI:10. 3969/j.issn.1001-5256.2017.08.035.
|
| [13] |
ZHOU LL, LIU N, LI CX, et al. Research advances in the diagnosis and treatment of patients in the immune tolerance stage of chronic hepatitis B virus infection[J]. J Clin Hepatol, 2019, 35(8): 1824-1827. DOI: 10.3969/j.issn.1001-5256.2019.08.039. |
| [14] |
LIU M, ZUO LL, ZHANG RY, et al. Research progress of antiviral therapy in patients with chronic HBV infection in immune tolerance phase[J]. Chin J Infect Dis, 2020, 38(11): 750-752. DOI: 10.3760/cma.j.cn311365-20190422-00138. |
| [15] |
ZHUANG H. Should patients in the immune tolerance stage of chronic hepatitis B virus infection be treated?[J]. J Clin Hepatol, 2021, 37(2): 272-277. DOI: 10.3969 /j.issn.1001-5256.2021.02.007.
庄辉. 慢性HBV感染免疫耐受期应否治疗?[J]. 临床肝胆病杂志, 2021, 37(2): 272-277. DOI: 10.3969 /j.issn.1001-5256.2021.02.007.
|
| [16] |
LOK AS, MCMAHON BJ, BROWN RS Jr, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis[J]. Hepatology, 2016, 63(1): 284-306. DOI: 10.1002/hep.28280. |
| [17] |
LEE HW, CHON YE, KIM BK, et al. Negligible HCC risk during stringently defined untreated immune-tolerant phase of chronic hepatitis B[J]. Eur J Intern Med, 2021, 84: 68-73. DOI: 10.1016/j.ejim.2020.10.022. |
| [18] |
KLAIR JS, VANCURA J, MURALI AR. PRO: Patients with chronic hepatitis B in immune-tolerant phase should be treated[J]. Clin Liver Dis (Hoboken), 2020, 15(1): 21-24. DOI: 10.1002/cld.892. |
| [19] |
ROCKEY DC, CALDWELL SH, GOODMAN ZD, et al. Liver biopsy[J]. Hepatology, 2009, 49(3): 1017-1044. DOI: 10.1002/hep.22742. |
| [20] |
Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B(2015 version)[J]. J Clin Hepatol, 2015, 31(12): 1941-1960. DOI: 10.3969/j.issn.1001-5256.2015.12.002. 中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2015年版)[J]. 临床肝胆病杂志, 2015, 31(12): 1941-1960. DOI: 10. 3969 /j. issn. 1001-5256. 2015. 12. 002.
|
| [21] |
ZHUANG H. Correction note on the estimated number of patients in the immune - tolerant phase of hepatitis B virus infection in China and globally[J]. J Clin Hepatol, 2021, 37(4): 785-786. DOI: 10.3969 /j.issn.1001-5256.2021.04.012.
|
| [22] |
Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: A modelling study[J]. Lancet Gastroenterol Hepatol, 2018, 3(6): 383-403. DOI: 10.1016/S2468-1253(18)30056-6. |
| [23] |
BERTOLETTI A, KENNEDY PT. The immune tolerant phase of chronic HBV infection: New perspectives on an old concept[J]. Cell Mol Immunol, 2015, 12(3): 258-263. DOI: 10.1038/cmi.2014.79. |
| [24] |
LIAW YF, CHU CM. Immune tolerance phase of chronic hepatitis B[J]. Gastroenterology, 2017, 152(5): 1245-1246. DOI: 10.1053/j.gastro.2016.11.057. |
| [25] |
KENNEDY PTF, BERTOLETTI A, MASON WS. Reply to immune tolerance phase of chronic hepatitis B[J]. Gastroenterology, 2017, 152(5): 1246-1247. DOI: 10.1053/j.gastro.2017.03.002. |
| [26] |
WANG H, XUE L, YAN R, et al. Comparison of FIB-4 and APRI in Chinese HBV-infected patients with persistently normal ALT and mildly elevated ALT[J]. J Viral Hepat, 2013, 20(4): e3-e10. DOI: 10.1111/jvh.12010. |
| [27] |
FATTOVICH G, BORTOLOTTI F, DONATO F. Natural history of chronic hepatitis B: Special emphasis on disease progression and prognostic factor[J]. J Hepatol, 2008, 48(2): 335-352. DOI: 10.1016/j.jhep.2007.11.011. |
| [28] |
RAFFETTI E, FATTOVICH G, DONATO F. Incidence of hepatocellular carcinoma in untreated subjects with chronic hepatitis B: A systematic review and meta-analysis[J]. Liver Int, 2016, 36(9): 1239-1251. DOI: 10.1111/liv.13142. |
| [29] |
ILOEJE UH, YANG HI, SU J, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load[J]. Gastroenterology, 2006, 130(3): 678-686. DOI: 10.1053/j.gastro.2005.11.016. |



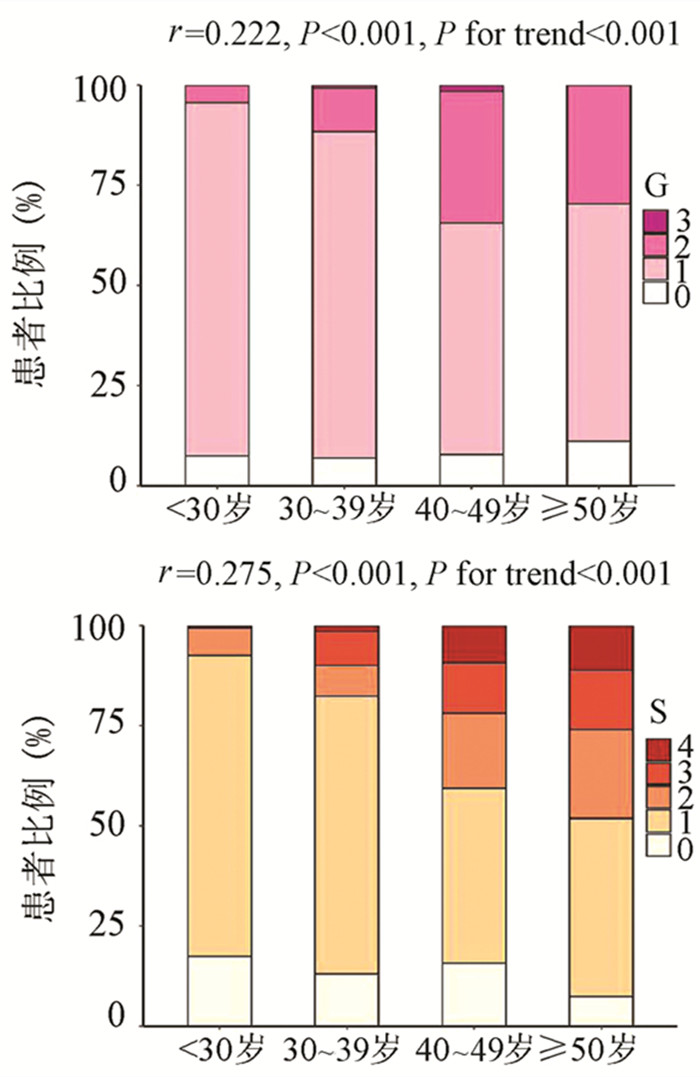




 DownLoad:
DownLoad: