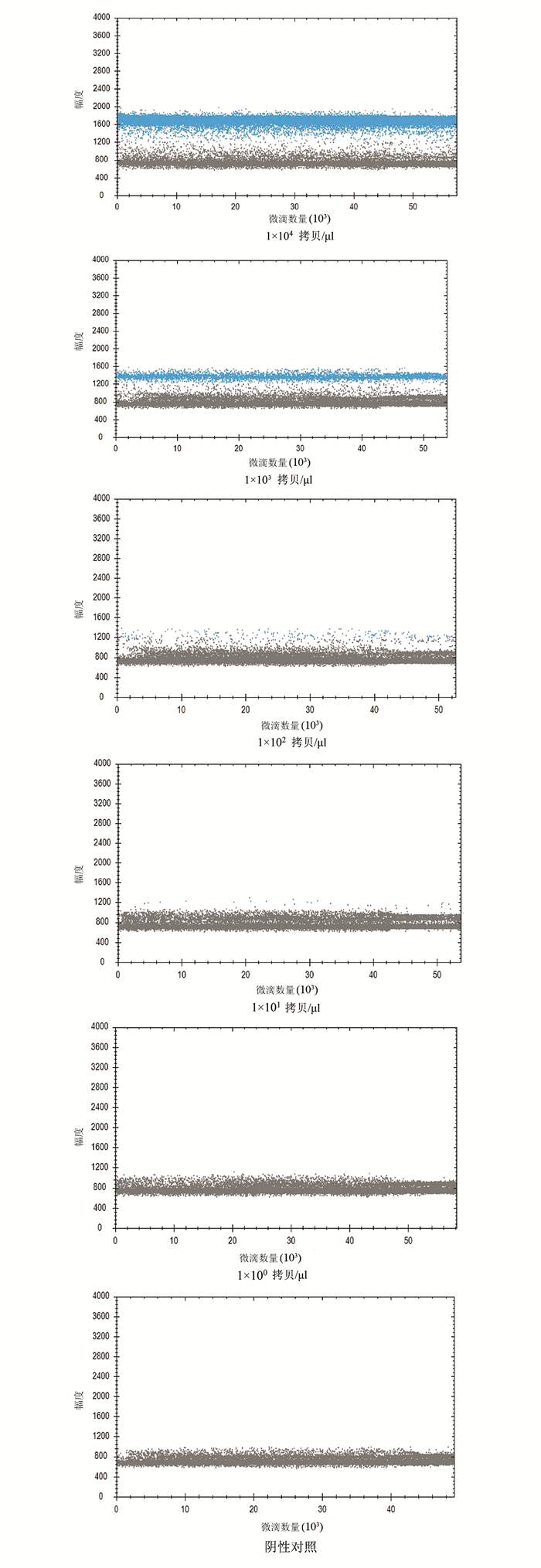
| [1] |
|
| [2] |
RAZAVI-SHEARER D, GAMKRELIDZE I, NGUYEN MH, et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: A modelling study[J]. Lancet Gastroenterol Hepatol, 2018, 3(6): 383-403. DOI: 10.1016/S2468-1253(18)30056-6. |
| [3] |
ALLWEISS L, VOLZ T, GIERSCH K, et al. Proliferation of primary human hepatocytes and prevention of hepatitis B virus reinfection efficiently deplete nuclear cccDNA in vivo[J]. Gut, 2018, 67(3): 542-552. DOI: 10.1136/gutjnl-2016-312162. |
| [4] |
LAI CL, WONG D, IP P, et al. Reduction of covalently closed circular DNA with long-term nucleos(t)ide analogue treatment in chronic hepatitis B[J]. J Hepatol, 2017, 66(2): 275-281. DOI: 10.1016/j.jhep.2016.08.022. |
| [5] |
NEWBOLD JE, XIN H, TENCZA M, et al. The covalently closed duplex form of the hepadnavirus genome exists in situ as a heterogeneous population of viral minichromosomes[J]. J Virol, 1995, 69(6): 3350-3357. DOI: 10.1128/JVI.69.6.3350-3357.1995. |
| [6] |
SEEGER C, MASON WS. Molecular biology of hepatitis B virus infection[J]. Virology, 2015, 479-480: 672-686. DOI: 10.1016/j.virol.2015.02.031. |
| [7] |
|
| [8] |
WERLE-LAPOSTOLLE B, BOWDEN S, LOCARNINI S, et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy[J]. Gastroenterology, 2004, 126(7): 1750-1758. DOI: 10.1053/j.gastro.2004.03.018. |
| [9] |
SI LL, LI XD, LI L, et al. Inhibitory effect of Suduxing extracts on covalently closed circular DNA of hepatitis B virus[J/CD]. Chin J Exp Clin Infect Dis(Electronic Edition), 2020, 14(4): 265-271. DOI: 10.3877/cma.j.issn.1674-1358.2020.04.001. |
| [10] |
TUTTLEMAN JS, POURCEL C, SUMMERS J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells[J]. Cell, 1986, 47(3): 451-460. DOI: 10.1016/0092-8674(86)90602-1. |
| [11] |
ZHONG Y, HAN J, ZOU Z, et al. Quantitation of HBV covalently closed circular DNA in micro formalin fixed paraffin-embedded liver tissue using rolling circle amplification in combination with real-time PCR[J]. Clin Chim Acta, 2011, 412(21-22): 1905-1911. DOI: 10.1016/j.cca.2011.06.031. |
| [12] |
XU CH, LI ZS, DAI JY, et al. Nested real-time quantitative polymerase chain reaction assay for detection of hepatitis B virus covalently closed circular DNA[J]. Chin Med J (Engl), 2011, 124(10): 1513-1516.
|
| [13] |
GUO Y, SHENG S, NIE B, et al. Development of magnetic capture hybridization and quantitative polymerase chain reaction for hepatitis B virus covalently closed circular DNA[J]. Hepat Mon, 2015, 15(1): e23729. DOI: 10.5812/hepatmon.23729. |
| [14] |
WHITE RA 3rd, QUAKE SR, CURR K. Digital PCR provides absolute quantitation of viral load for an occult RNA virus[J]. J Virol Methods, 2012, 179(1): 45-50. DOI: 10.1016/j.jviromet.2011.09.017. |
| [15] |
HINDSON BJ, NESS KD, MASQUELIER DA, et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number[J]. Anal Chem, 2011, 83(22): 8604-8610. DOI: 10.1021/ac202028g. |
| [16] |
VOGELSTEIN B, KINZLER KW. Digital PCR[J]. Proc Natl Acad Sci U S A, 1999, 96(16): 9236-9241. DOI: 10.1073/pnas.96.16.9236. |
| [17] |
JAHNE MA, BRINKMAN NE, KEELY SP, et al. Droplet digital PCR quantification of norovirus and adenovirus in decentralized wastewater and graywater collections: Implications for onsite reuse[J]. Water Res, 2020, 169: 115213. DOI: 10.1016/j.watres.2019.115213. |
| [18] |
PAN Y, MA T, MENG Q, et al. Droplet digital PCR enabled by microfluidic impact printing for absolute gene quantification[J]. Talanta, 2020, 211: 120680. DOI: 10.1016/j.talanta.2019.120680. |
| [19] |
YU F, YAN L, WANG N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients[J]. Clin Infect Dis, 2020, 71(15): 793-798. DOI: 10.1093/cid/ciaa345. |
| [20] |
PROFAIZER T, SLEV P. A multiplex, droplet digital pcr assay for the detection of T-cell receptor excision circles and kappa-deleting recombination excision circles[J]. Clin Chem, 2020, 66(1): 229-238. DOI: 10.1373/clinchem.2019.308171. |
| [21] |
CAVIGLIA GP, ABATE ML, TANDOIF, et al. Quantitation of HBV cccDNA in anti-HBc-positive liver donors by droplet digital PCR: A new tool to detect occult infection[J]. J Hepatol, 2018, 69(2): 301-307. DOI: 10.1016/j.jhep.2018.03.021. |



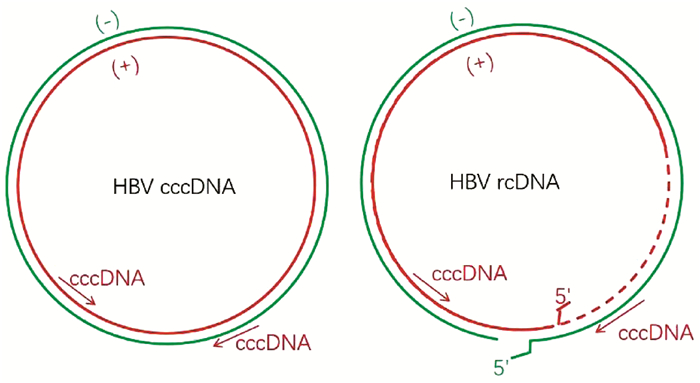




 DownLoad:
DownLoad: