| [1] |
BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492. |
| [2] |
|
| [3] |
PATRA KC, HAY N. The pentose phosphate pathway and cancer[J]. Trends Biochem Sci, 2014, 39(8): 347-354. DOI: 10.1016/j.tibs.2014.06.005. |
| [4] |
CAI T, KUANG Y, ZHANG C, et al. Glucose-6-phosphate dehydrogenase and NADPH oxidase 4 control STAT3 activity in melanoma cells through a pathway involving reactive oxygen species, c-SRC and SHP2[J]. Am J Cancer Res, 2015, 5(5): 1610-1620. http://europepmc.org/articles/PMC4497430 |
| [5] |
MELE L, LA NOCE M, PAINO F, et al. Glucose-6-phosphate dehydrogenase blockade potentiates tyrosine kinase inhibitor effect on breast cancer cells through autophagy perturbation[J]. J Exp Clin Cancer Res, 2019, 38(1): 160. DOI: 10.1186/s13046-019-1164-5. |
| [6] |
ZHANG R, TAO F, RUAN S, et al. The TGFβ1-FOXM1-HMGA1-TGFβ1 positive feedback loop increases the cisplatin resistance of non-small cell lung cancer by inducing G6PD expression[J]. Am J Transl Res, 2019, 11(11): 6860-6876. http://www.ncbi.nlm.nih.gov/pubmed/31814893 |
| [7] |
RAO X, DUAN X, MAO W, et al. O-GlcNAcylation of G6PD promotes the pentose phosphate pathway and tumor growth[J]. Nat Commun, 2015, 6: 8468. DOI: 10.1038/ncomms9468. |
| [8] |
BARAJAS JM, REYES R, GUERRERO MJ, et al. The role of miR-122 in the dysregulation of glucose-6-phosphate dehydrogenase (G6PD) expression in hepatocellular cancer[J]. Sci Rep, 2018, 8(1): 9105. DOI: 10.1038/s41598-018-27358-5. |
| [9] |
PES GM, ERRIGO A, SORO S, et al. Glucose-6-phosphate dehydrogenase deficiency reduces susceptibility to cancer of endodermal origin[J]. Acta Oncol, 2019, 58(9): 1205-1211. DOI: 10.1080/0284186X.2019.1616815. |
| [10] |
ZHANG X, ZHANG X, LI Y, et al. PAK4 regulates G6PD activity by p53 degradation involving colon cancer cell growth[J]. Cell Death Dis, 2017, 8(5): e2820. DOI: 10.1038/cddis.2017.85. |
| [11] |
TANG Y, HU HQ, TANG FX, et al. Combined preoperative LMR and CA125 for prognostic assessment of ovarian cancer[J]. J Cancer, 2020, 11(11): 3165-3171. DOI: 10.7150/jca.42477. |
| [12] |
MANDALIYA H, JONES M, OLDMEADOW C, et al. Prognostic biomarkers in stage Ⅳ non-small cell lung cancer (NSCLC): Neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI)[J]. Transl Lung Cancer Res, 2019, 8(6): 886-894. DOI: 10.21037/tlcr.2019.11.16. |
| [13] |
ISMAEL MN, FORDE J, MILLA E, et al. Utility of inflammatory markers in predicting hepatocellular carcinoma survival after liver transplantation[J]. Biomed Res Int, 2019, 2019: 7284040. DOI: 10.1155/2019/7284040. |
| [14] |
Bureau of Medical Administration, National Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)[J]. J Clin Hepatol, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007. |
| [15] |
European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma[J]. J Hepatol, 2018, 69(1): 182-236. DOI: 10.1016/j.jhep.2018.03.019. |
| [16] |
SHANG RZ, QU SB, WANG DS. Reprogramming of glucose metabolism in hepatocellular carcinoma: Progress and prospects[J]. World J Gastroenterol, 2016, 22(45): 9933-9943. DOI: 10.3748/wjg.v22.i45.9933. |
| [17] |
ZUCMAN-ROSSI J, VILLANUEVA A, NAULT JC, et al. Genetic landscape and biomarkers of hepatocellular carcinoma[J]. Gastroenterology, 2015, 149(5): 1226-1239. e4. DOI: 10.1053/j.gastro.2015.05.061. |
| [18] |
LU M, LU L, DONG Q, et al. Elevated G6PD expression contributes to migration and invasion of hepatocellular carcinoma cells by inducing epithelial-mesenchymal transition[J]. Acta Biochim Biophys Sin (Shanghai), 2018, 50(4): 370-380. DOI: 10.1093/abbs/gmy009. |
| [19] |
SOSA V, MOLINÉ T, SOMOZA R, et al. Oxidative stress and cancer: An overview[J]. Ageing Res Rev, 2013, 12(1): 376-390. DOI: 10.1016/j.arr.2012.10.004. |
| [20] |
|
| [21] |
YAN YC, WEN K, MAO K, et al. Pathogenesis of hepatitis B virus-related hepatocellular carcinoma[J]. J Clin Hepatol, 2020, 36(10): 2167-2172. DOI: 10.3969/j.issn.1001-5256.2020.10.002. |
| [22] |
LIU B, FANG M, HE Z, et al. Hepatitis B virus stimulates G6PD expression through HBx-mediated Nrf2 activation[J]. Cell Death Dis, 2015, 6: e1980. DOI: 10.1038/cddis.2015.322. |
| [23] |
LIM CJ, LEE YH, PAN L, et al. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma[J]. Gut, 2019, 68(5): 916-927. DOI: 10.1136/gutjnl-2018-316510. |
| [24] |
CHEN XQ, XUE CR, HOU P, et al. Lymphocyte-to-monocyte ratio effectively predicts survival outcome of patients with obstructive colorectal cancer[J]. World J Gastroenterol, 2019, 25(33): 4970-4984. DOI: 10.3748/wjg.v25.i33.4970. |
| [25] |
ZHU YW, YAO MJ, BO YC, et al. Prognostic assessment of lymphocyte-to-monocyte ratio for patients with primary liver cancer after curative hepatectomy[J]. J Zhengzhou Univ (Medical Sciences), 2017, 52(2): 197-200. DOI: 10.13705/j.issn.1671-6825.2017.02.023. |
| [26] |
MANO Y, YOSHIZUMI T, YUGAWA K, et al. Lymphocyte-to-monocyte ratio is a predictor of survival after liver transplantation for hepatocellular carcinoma[J]. Liver Transpl, 2018, 24(11): 1603-1611. DOI: 10.1002/lt.25204. |



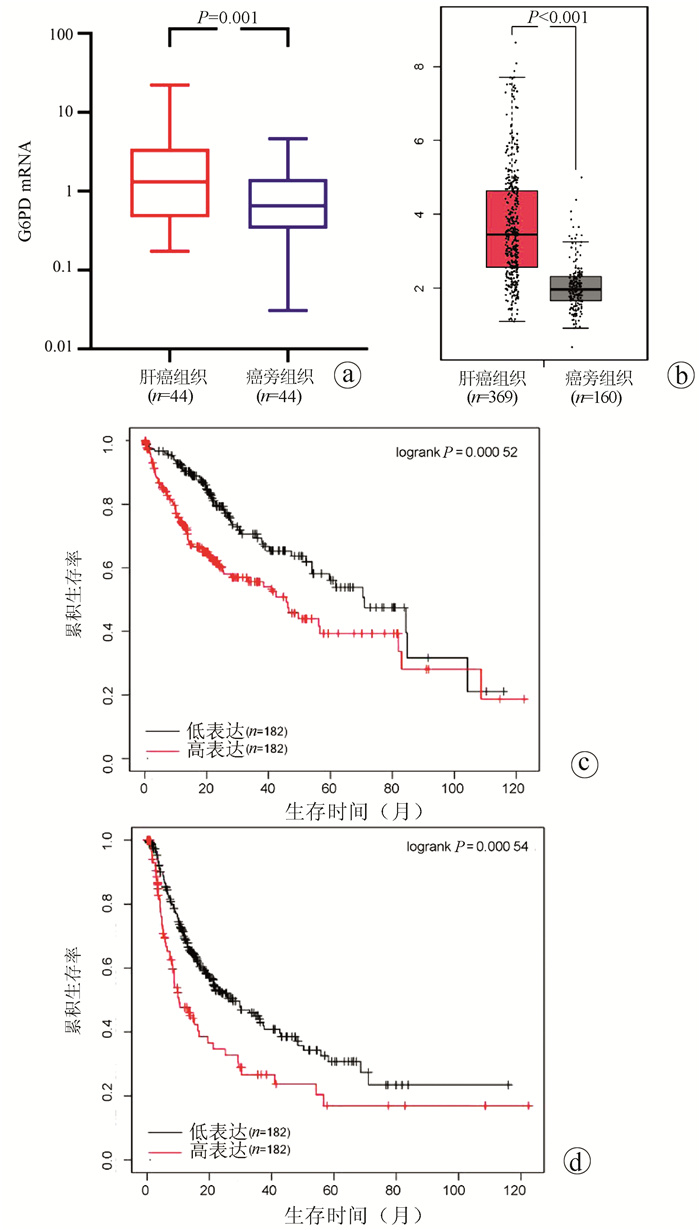




 DownLoad:
DownLoad:
