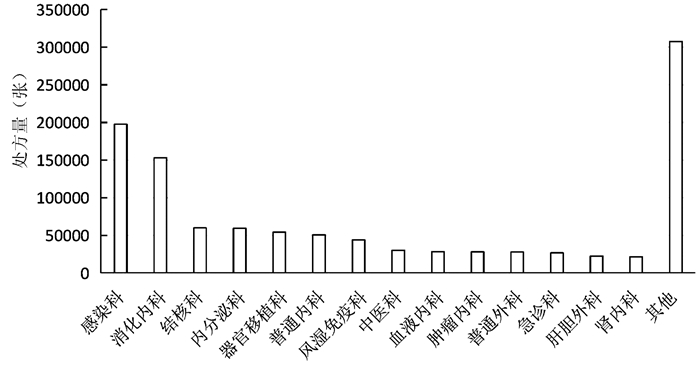
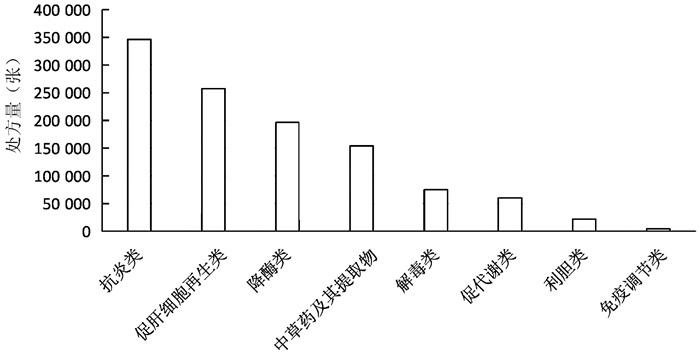
| [1] |
|
| [2] |
|
| [3] |
LIU JY, ZHANG CL, LI XP, et al. Utilization analysis of hepatoprotective drugs of 34 hospitals in Wuhan area during 2012-2014[J]. Chin J Pharmacoepidemiol, 2016, 25(2): 113-118. DOI: CNKI:SUN:YWLX.0.2016-02-012. |
| [4] |
|
| [5] |
Chinese Society of Infectious Diseases, Expert Consensus Committee on Liver Inflammation and Its Prevention and Treatment. Prevention and management of liver inflammation: An expert consensus in China[J]. Chin J Clin Infect Dis, 2014, 7(1): 4-12. DOI: 10.3760/cma.j.issn.1674-2397.2014.01.002. |
| [6] |
CAO M, LI X, ZHANG B, et al. The effect of polyene phosphatidyl choline intervention on nonalcoholic steatohepatitis and related mechanism[J]. Am J Transl Res, 2016, 8(5): 2325-2330.
|
| [7] |
WEN XL, ZHANG HL. Literature anaiysis of adverse reactions caused by poiyene phosphatidyi choiine [J]. Chin J Pharmacov, 2020, 17(8): 526-530. DOI: 10.19803/j.1672-8629.2020.08.16. |
| [8] |
YANG YL, ZHANG ZQ, WEI YQ. Clinical study on silybinin combined with polyene phosphatidylcholine for nonalcoholic fatty liver disease[J]. J New Chin Med, 2020, 52(18): 81-84. DOI: 10.13457/j.cnki.jncm.2020.19.023. |
| [9] |
CHEN JH, XU YM. Clinical effect of polyene phosphatidylcholine combined with metformin in the treatment of non-alcoholic fatty liver disease [J]. Chronic Pathematol J, 2020, 21(6): 885-886. DOI: 10.16440/j.cnki.1674-8166.2020.06.031. |
| [10] |
CHENG ZH. Clinical effect of polyene phosphatidylcholine combined with reduced glutathione in the treatment of alcoholic hepatitis [J]. Chin J Med Devi, 2020, 33(19): 115-116. DOI: 10.14163/J.CNKI.11-5547/R.2015.09.112. |
| [11] |
LI CY, SHI YH. Effect of polyene phosphatidylcholine on liver fibrosis and liver function of alcoholic fatty liver[J]. Hainan Med J, 2020, 31(13): 1649-1651. DOI: 10.3969/j.issn.1003-6350.2020.13.004. |
| [12] |
XU H, GAO ZW, LIU JX, et al. Effect of polyene phosphatidylcholine on liver fibrosis and liver function of alcoholic fatty liver [J]. Hainan Med J, 2018, 15(4): 164-167. DOI: 10.3969/j.issn.1003-6350.2020.13.004. |
| [13] |
SHI L. Analysis of the effects of polyene phosphatidylcholine combined with silybin on liver function and blood lipid levels in patients with chronic hepatitis B[J]. Chin J Pharm Econom, 2020, 15(5): 94-97. DOI: 10.12010/j.issn.1673-5846.2020.05.021. |
| [14] |
SHANG YH. Efficacy of bicyclol combined with polyene phosphatidylcholine in the treatment of hyperthyroid liver injury [J]. Chin J Modern Drug Appl, 2020, 14(4): 9-11. DOI: 10.14164/j.cnki.cn11-5581/r.2020.04.004. |
| [15] |
XIE W, YU YC. Expert consensus on clinical application of bicyclol—2020 edition [J/CD]. Chin J Exp Clin Infect Dis (Electronic Edition), 2020, 14(3): 177-185. DOI: 10.3877/cma.j.issn.1674-1358.2020.03.001. |
| [16] |
LI H, LI JR, HUANG MH, et al. Bicyclol attenuates liver inflammation induced by infection of hepatitis C virus via repressing ROS-mediated activation of MAPK/NF-κB signaling pathway[J]. Front Pharmacol, 2018, 9: 1438. DOI: 10.3389/fphar.2018.01438. |
| [17] |
|
| [18] |
DU DF, WANG RZ. Efficacy of compound glycyrrhizin with narrow-band ultraviolet radiation in the treatment of psoriasis vulgaris and its effect on cytokines and proliferation genes[J]. J Clin Exp Med, 2020, 19(3): 307-310. DOI: 10.3969/j.issn.1671-4695.2020.03.024. |
| [19] |
|
| [20] |
National Healthcare Security Administration, Ministry of Human Resources and Social Security of the People's Republic of China. "National Basic Medical Insurance, Industrial Injury Insurance and Maternity Insurance Drug Catalogue (2020)" [EB/OL]. [2020-12-25] (2021-03-30). http://www.nhsa.gov.cn/art/2019/8/20/art_37_1666.html |








 DownLoad:
DownLoad: