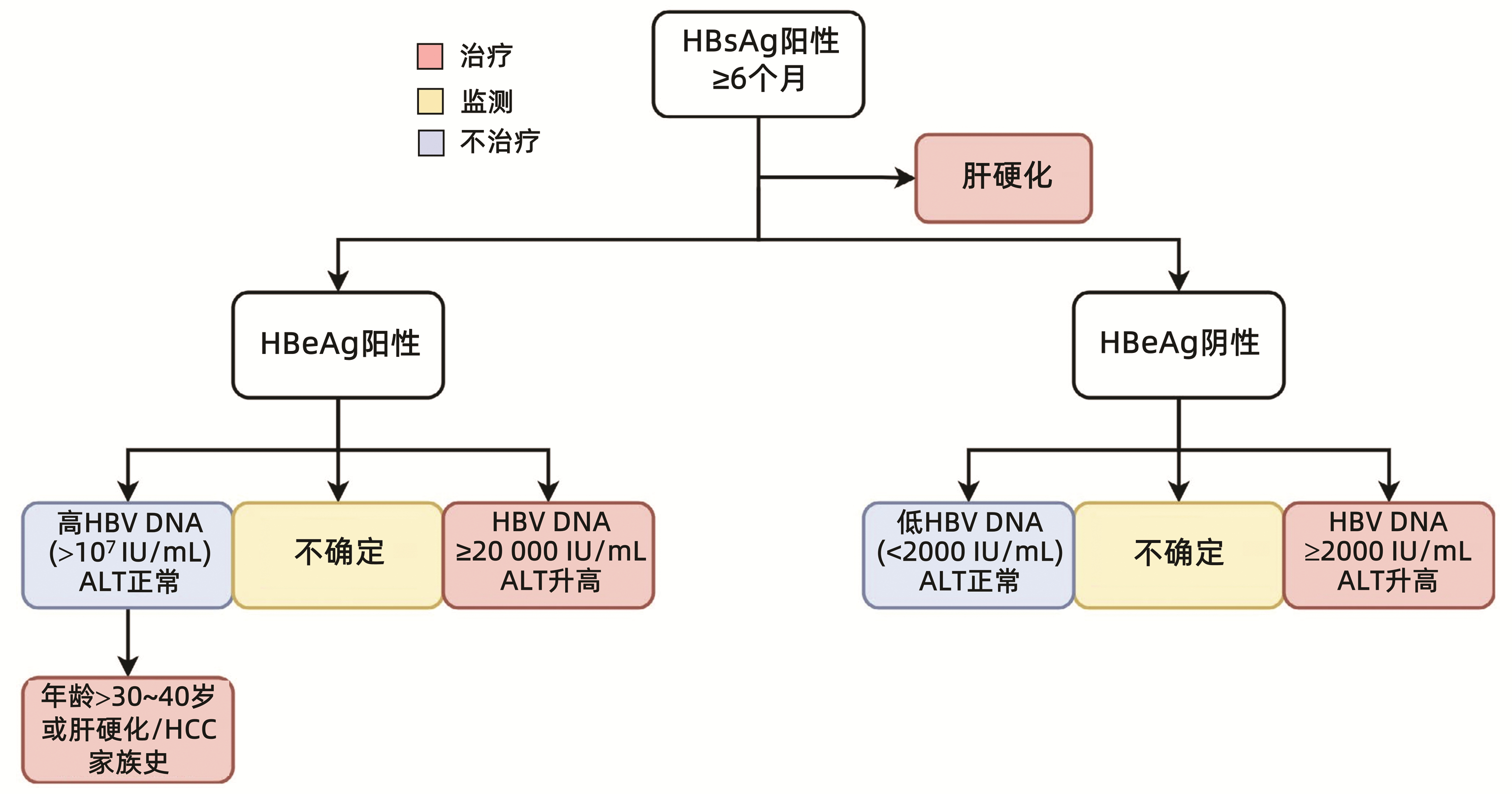
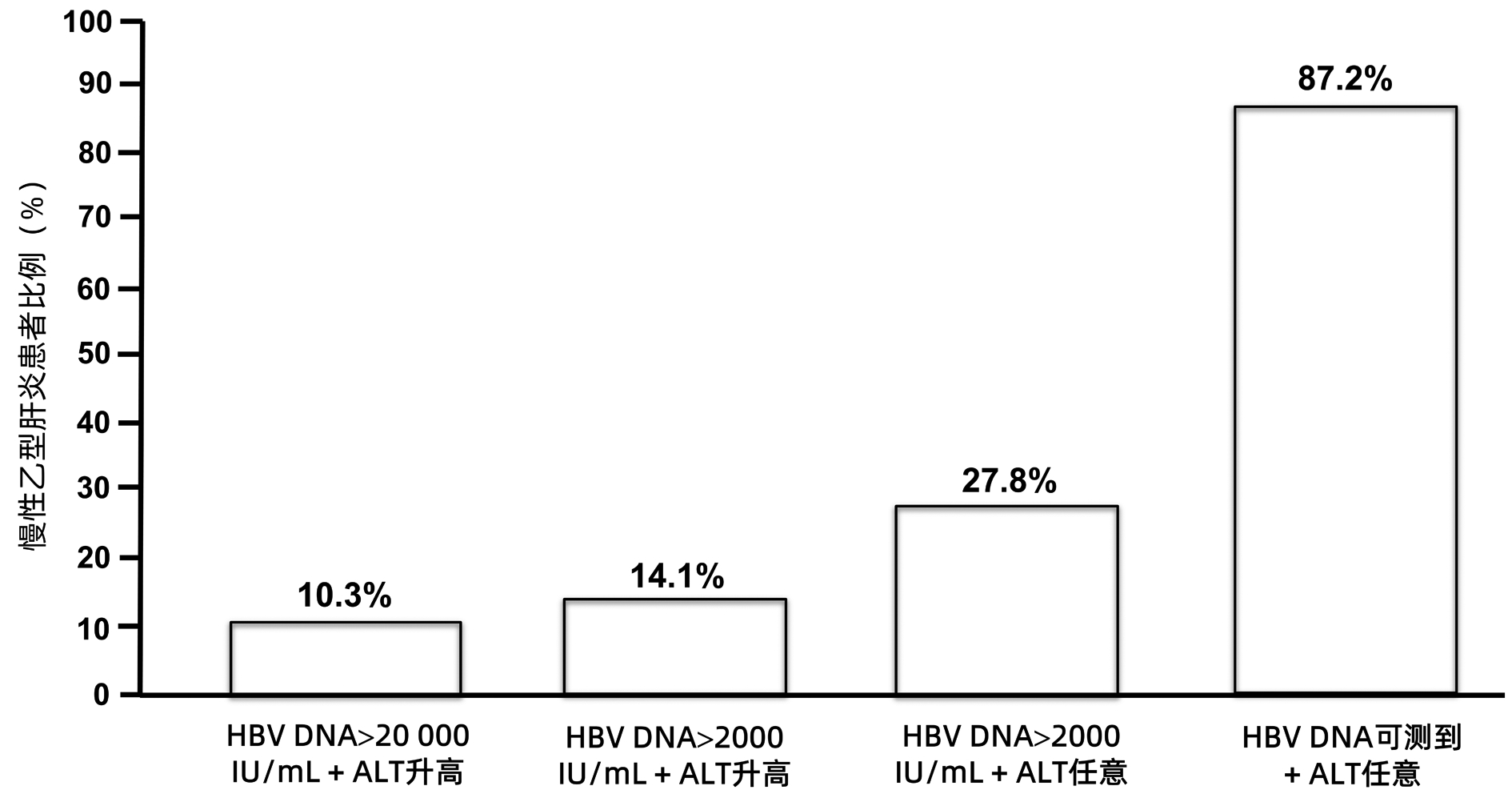
| [1] |
SORIANO V, ALVAREZ C, EDAGWA B, et al. Ultra-long-acting (XLA) antivirals for chronic viral hepatitis[J]. Int J Infect Dis, 2022, 114: 45-50. DOI: 10.1016/j.ijid.2021.10.052. |
| [2] |
World Health Organization. Global hepatitis report, 2017[R]. Geneva: World Health Organization, 2017.
|
| [3] |
World Health Organization. Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022-2030[R]. Geneva: World Health Organization, 2022.
|
| [4] |
|
| [5] |
SINN DH, KIM SE, KIM BK, et al. The risk of hepatocellular carcinoma among chronic hepatitis B virus-infected patients outside current treatment criteria[J]. J Viral Hepat, 2019, 26(12): 1465-1472. DOI: 10.1111/jvh.13185. |
| [6] |
SARIN SK, KUMAR M, LAU GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update[J]. Hepatol Int, 2016, 10(1): 1-98. DOI: 10.1007/s12072-015-9675-4. |
| [7] |
TERRAULT NA, LOK A, MCMAHON BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance[J]. Hepatology, 2018, 67(4): 1560-1599. DOI: 10.1002/hep.29800. |
| [8] |
European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection[J]. J Hepatol, 2017, 67(2): 370-398. DOI: 10.1016/j.jhep.2017.03.021. |
| [9] |
PAPATHEODORIDIS GV, LAMPERTICO P, MANOLAKOPOULOS S, et al. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review[J]. J Hepatol, 2010, 53(2): 348-356. DOI: 10.1016/j.jhep.2010.02.035. |
| [10] |
PAPATHEODORIDIS GV, IDILMAN R, DALEKOS GN, et al. The risk of hepatocellular carcinoma decreases after the first 5 years of entecavir or tenofovir in Caucasians with chronic hepatitis B[J]. Hepatology, 2017, 66(5): 1444-1453. DOI: 10.1002/hep.29320. |
| [11] |
CHEN YC, CHU CM, LIAW YF. Age-specific prognosis following spontaneous hepatitis B e antigen seroconversion in chronic hepatitis B[J]. Hepatology, 2010, 51(2): 435-444. DOI: 10.1002/hep.23348. |
| [12] |
YUEN MF, WONG DK, FUNG J, et al. HBsAg seroclearance in chronic hepatitis B in Asian patients: replicative level and risk of hepatocellular carcinoma[J]. Gastroenterology, 2008, 135(4): 1192-1199. DOI: 10.1053/j.gastro.2008.07.008. |
| [13] |
CHOI H, TONTHAT A, JANSSEN H, et al. Aiming for functional cure with established and novel therapies for chronic hepatitis B[J]. Hepatol Commun, 2022, 6(5): 935-949. DOI: 10.1002/hep4.1875. |
| [14] |
WONG RJ, KAUFMAN HW, NILES JK, et al. Simplifying treatment criteria in chronic hepatitis B: Reducing barriers to elimination[J]. Clin Infect Dis, 2022. DOI: 10.1093/cid/ciac385.[Onlineaheadofprint] |
| [15] |
TONG MJ, PAN CQ, HAN SB, et al. An expert consensus for the management of chronic hepatitis B in Asian Americans[J]. Aliment Pharmacol Ther, 2018, 47(8): 1181-1200. DOI: 10.1111/apt.14577. |
| [16] |
RAZAVI-SHEARER D, ESTES C, GAMKRELIDZE I, et al. Cost-effectiveness analysis of treating all HBsAg+ individuals in the United States[J]. Hepatology, 2021, 74(Suppl 1): 22A.
|
| [17] |
LIM YS, AHN SH, SHIM JJ, et al. Impact of expanding hepatitis B treatment guidelines: A modelling and economic impact analysis[J]. Aliment Pharmacol Ther, 2022, 56(3): 519-528. DOI: 10.1111/apt.17052. |
| [18] |
Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study[J]. Lancet Gastroenterol Hepatol, 2018, 3(6): 383-403. DOI: 10.1016/S2468-1253(18)30056-6. |
| [19] |
TOY M, HUTTON D, HARRIS AM, et al. Cost-effectiveness of 1-time universal screening for chronic hepatitis B infection in adults in the United States[J]. Clin Infect Dis, 2022, 74(2): 210-217. DOI: 10.1093/cid/ciab405. |
| [20] |
RAMRAKHIANI NS, CHEN VL, LE M, et al. Optimizing hepatitis B virus screening in the United States using a simple demographics-based model[J]. Hepatology, 2022, 75(2): 430-437. DOI: 10.1002/hep.32142. |
| [21] |
SU S, WONG WC, ZOU Z, et al. Cost-effectiveness of universal screening for chronic hepatitis B virus infection in China: an economic evaluation[J]. Lancet Glob Health, 2022, 10(2): e278-e287. DOI: 10.1016/S2214-109X(21)00517-9. |
| [22] |
WENG MK, DOSHANI M, KHAN MA, et al. Universal hepatitis B vaccination in adults aged 19-59 years: Updated recommendations of the advisory committee on immunization practices-United States, 2022[J]. MMWR Morb Mortal Wkly Rep, 2022, 71(13): 477-483. DOI: 10.15585/mmwr.mm7113a1. |








 DownLoad:
DownLoad: