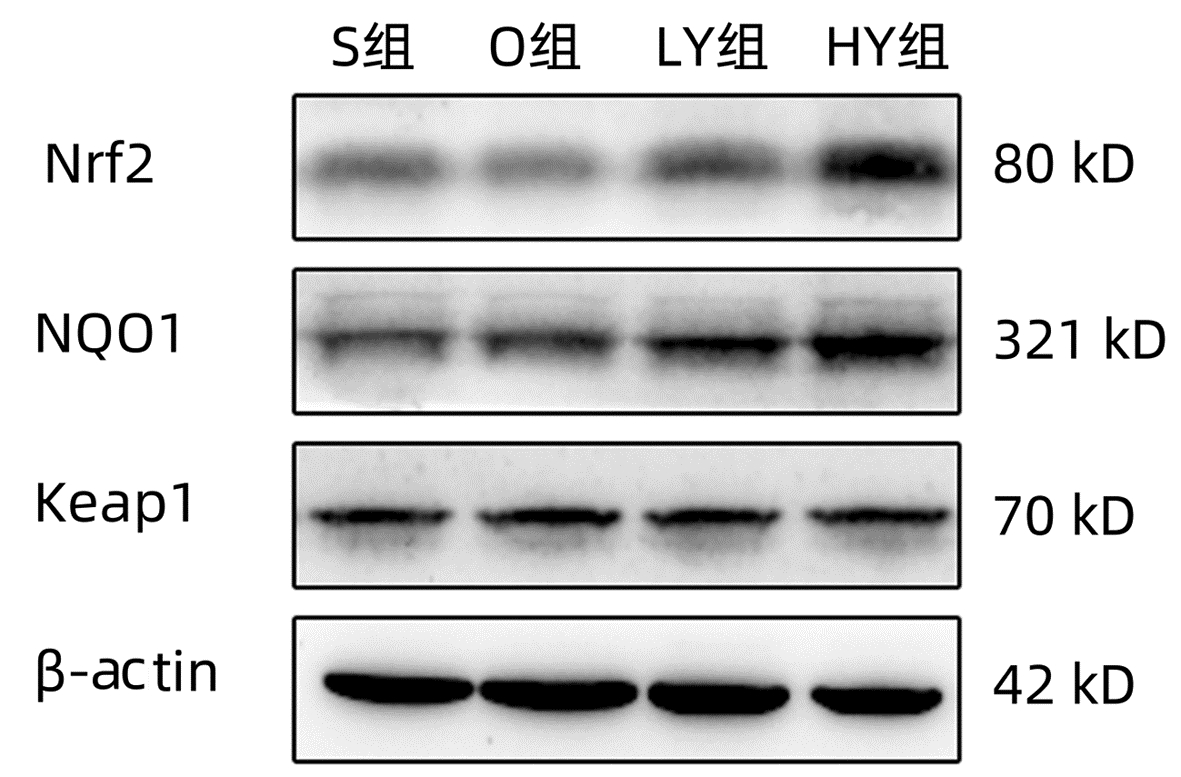
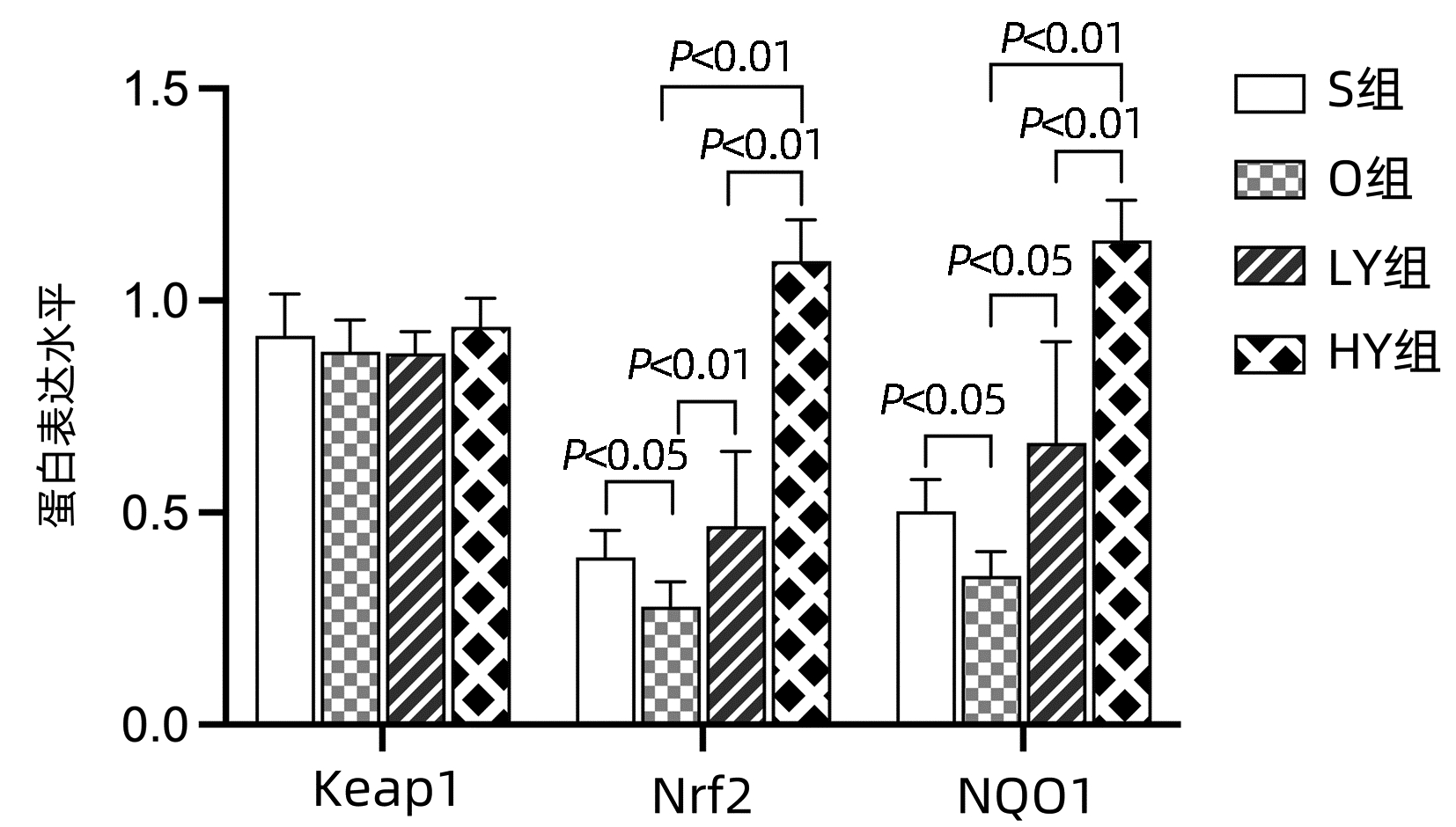
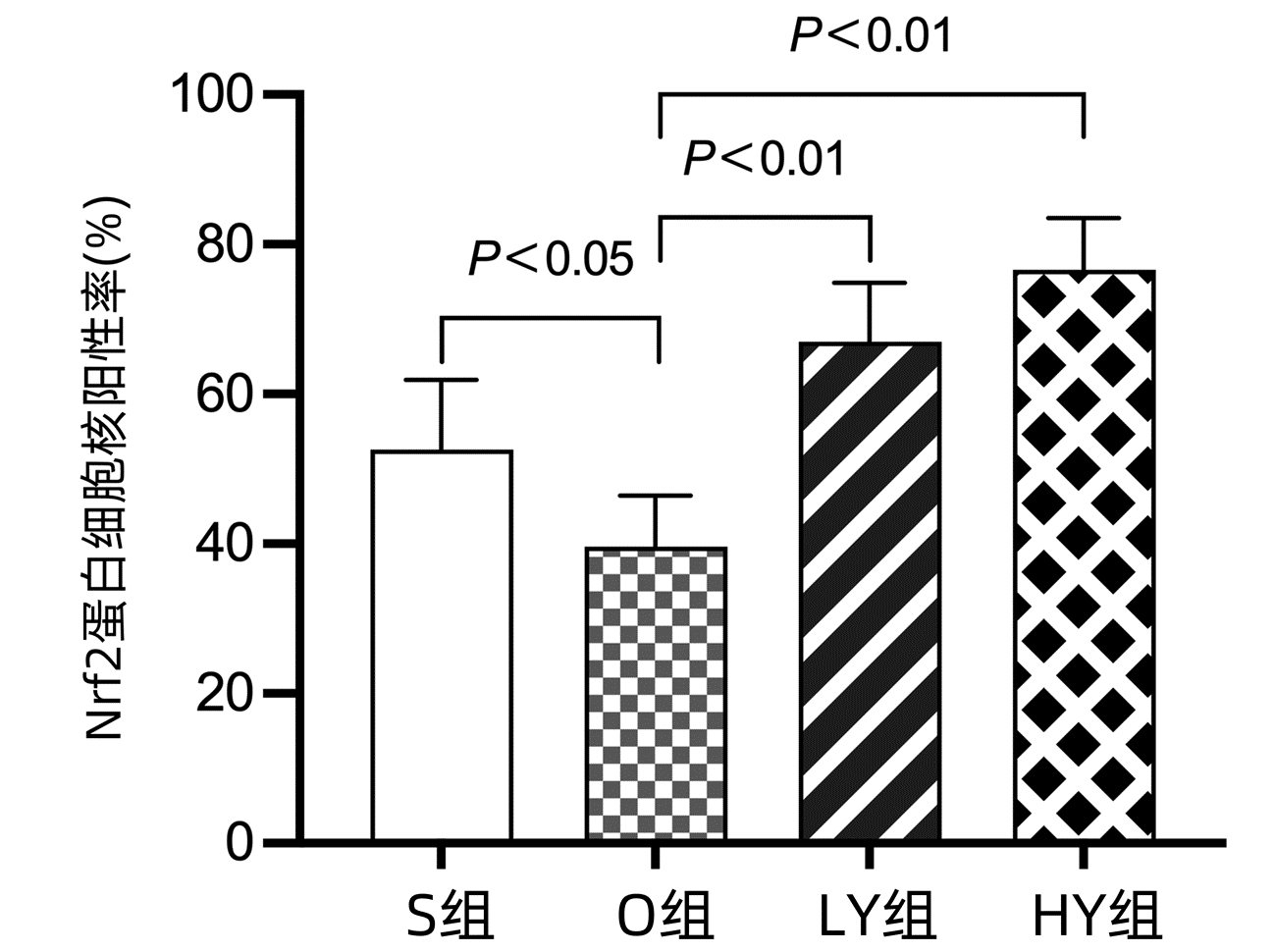
| [1] |
MARTÍNEZ-CECILIA D, REYES-DÍAZ M, RUIZ-RABELO J, et al. Oxidative stress influence on renal dysfunction in patients with obstructive jaundice: A case and control prospective study[J]. Redox Biol, 2016, 8: 160-164. DOI: 10.1016/j.redox.2015.12.009. |
| [2] |
LU XL, JIANG YY, CAO Q. The role of oxidative stress and nuclear factor erythroid 2-related factor 2 in nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2020, 36(4): 924-927. DOI: 10.3969/j.issn.1001-5256.2020.04.048. |
| [3] |
TURPAEV KT. Keap1-Nrf2 signaling pathway: mechanisms of regulation and role in protection of cells against toxicity caused by xenobiotics and electrophiles[J]. Biochemistry (Mosc), 2013, 78(2): 111-126. DOI: 10.1134/S0006297913020016. |
| [4] |
CAI GS, WEI ZH, XIAO HM, et al. Study on the dose-efficacy relationship of Yinchenhao decoction in past dynasties[J]. J Liaoning Univ Tradit Chin Med, 2019, 21(1): 129-132. DOI: 10.13194/j.issn.1673-842x.2019.01.035. |
| [5] |
LIU J, QU J, CHEN H, et al. The pathogenesis of renal injury in obstructive jaundice: A review of underlying mechanisms, inducible agents and therapeutic strategies[J]. Pharmacol Res, 2021, 163: 105311. DOI: 10.1016/j.phrs.2020.105311. |
| [6] |
FAHMY SR. Anti-fibrotic effect of Holothuria arenicola extract against bile duct ligation in rats[J]. BMC Complement Altern Med, 2015, 15: 14. DOI: 10.1186/s12906-015-0533-7. |
| [7] |
HUANG LP, XU YH, DENG MZ, et al. Research progress on chemical constituents, pharmacological mechanism and clinical application of Artemisiae Scopariae Herba[J]. Nat Prod Res Dev, 2021, 33(4): 676-690. DOI: 10.16333/j.1001-6880.2021.4.018. |
| [8] |
JIN LX, JIN LJ, LUAN ZQ, et al. Research progress on chemical constituents and pharmacology of rhubarb[J]. Inf Tradit Chin Med, 2020, 37(1): 121-126. DOI: 10.19656/j.cnki.1002-2406.200027. |
| [9] |
SHI YP, KONG HT, LI HN, et al. Research progress on chemical composition and pharmacological effects of Gardenia jasminoides and predictive analysis on quality marker (Q-marker)[J]. Chin Tradit Herb Drug, 2019, 50(2): 281-289. DOI: 10.7501/j.issn.0253-2670.2019.02.003. |
| [10] |
LIU JJ, LI ZL, SHANG HT. Study on Yinchenhao decoction regulating bile acid metabolism and intervening the mitochondrial DNA damage in liver cells of rats with obstructive jaundice[J]. Tianjin Med J, 2020, 48(9): 839-842. DOI: 10.11958/20200487. 刘军舰, 李忠廉, 尚海涛. 茵陈蒿汤调节胆汁酸代谢并干预阻塞性黄疸大鼠肝细胞线粒体DNA损伤的研究[J]. 天津医药, 2020, 48(9): 839-842. DOI: 10.11958/20200487. |
| [11] |
ZHANG XB, DUAN QL, LI ZL, et al. Expression of iNOS in the liver of rats with acute biliary obstruction[J]. Shandong Med J, 2013, 53(29): 19-21, 24. DOI: 10.3969/j.issn.1002-266X.2013.29.007. |
| [12] |
ZHANG HY, ZHAO B, WANG YH, et al. Emodin inhibits inflammation and oxidative stress by regulating Nrf2/HO-1 and MAPKs[J]. Chin J Immunol, 2021, 37(9): 1063-1068. DOI: 10.3969/j.issn.1000-484X.2021.09.008. |
| [13] |
TAGUCHI K, YAMAMOTO M. The KEAP1-NRF2 system as a molecular target of cancer treatment[J]. Cancers (Basel), 2020, 13(1): 46. DOI: 10.3390/cancers13010046. |
| [14] |
ZHOU J, WANG ZC, YIN YH. Discussion on prevention and treatment of diabetic nephropathy with Yiqi Huoxue method based on KEAP1-NRF2-ARE signaling pathway[J]. China J Chin Med, 2022, 37(4): 742-750. DOI: 10.16368/j.issn.1674-8999.2022.04.139. |
| [15] |
WANG D, LIU J, ZHANG XY, et al. Geniposide alleviates liver injuries in rats with severe pancreatitis through the Nrf2/ Keap1/ARE pathway[J]. J Changchun Univ Chin Med, 2022, 38(6): 631-635. DOI: 10.13463/j.cnki.cczyy.2022.06.011. |
| [16] |
BELLEZZA I, GIAMBANCO I, MINELLI A, et al. Nrf2-Keap1 signaling in oxidative and reductive stress[J]. Biochim Biophys Acta Mol Cell Res, 2018, 1865(5): 721-733. DOI: 10.1016/j.bbamcr.2018.02.010. |
| [17] |
HU LF, WANG Y, REN RJ, et al. Anti-oxidative stress actions and regulation mechanisms of Keap1-Nrf2/ARE signal pathway[J]. J Int Pharm Res, 2016, 43(1): 146-152, 166. DOI: 10.13220/j.cnki.jipr.2016.01.022. |
| [18] |
LU M, WANG P, QIAO Y, et al. GSK3β-mediated Keap1-independent regulation of Nrf2 antioxidant response: A molecular rheostat of acute kidney injury to chronic kidney disease transition[J]. Redox Biol, 2019, 26: 101275. DOI: 10.1016/j.redox.2019.101275. |
| [19] |
MA H, YANG B, YU L, et al. Sevoflurane protects the liver from ischemia-reperfusion injury by regulating Nrf2/HO-1 pathway[J]. Eur J Pharmacol, 2021, 898: 173932. DOI: 10.1016/j.ejphar.2021.173932. |



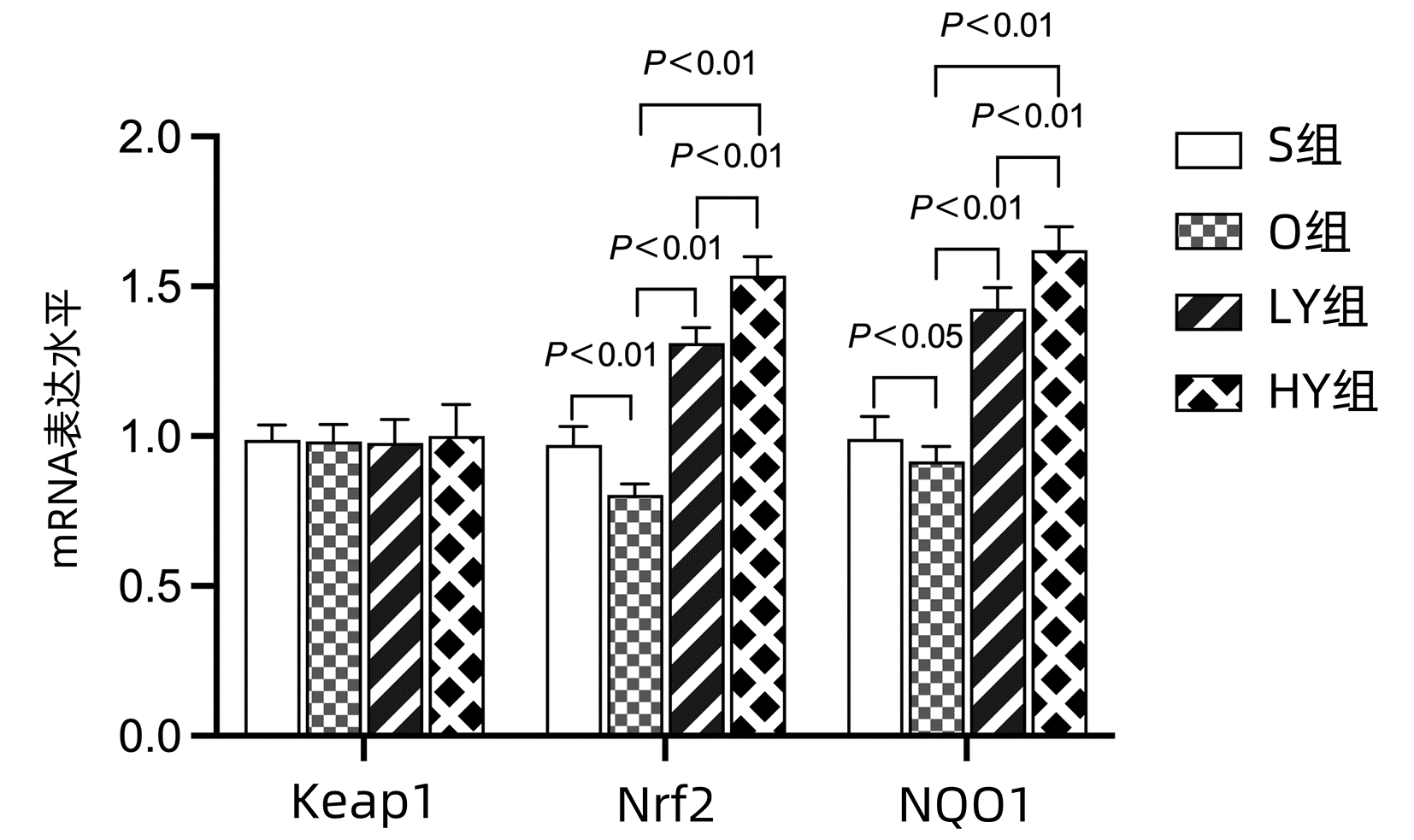




 DownLoad:
DownLoad: