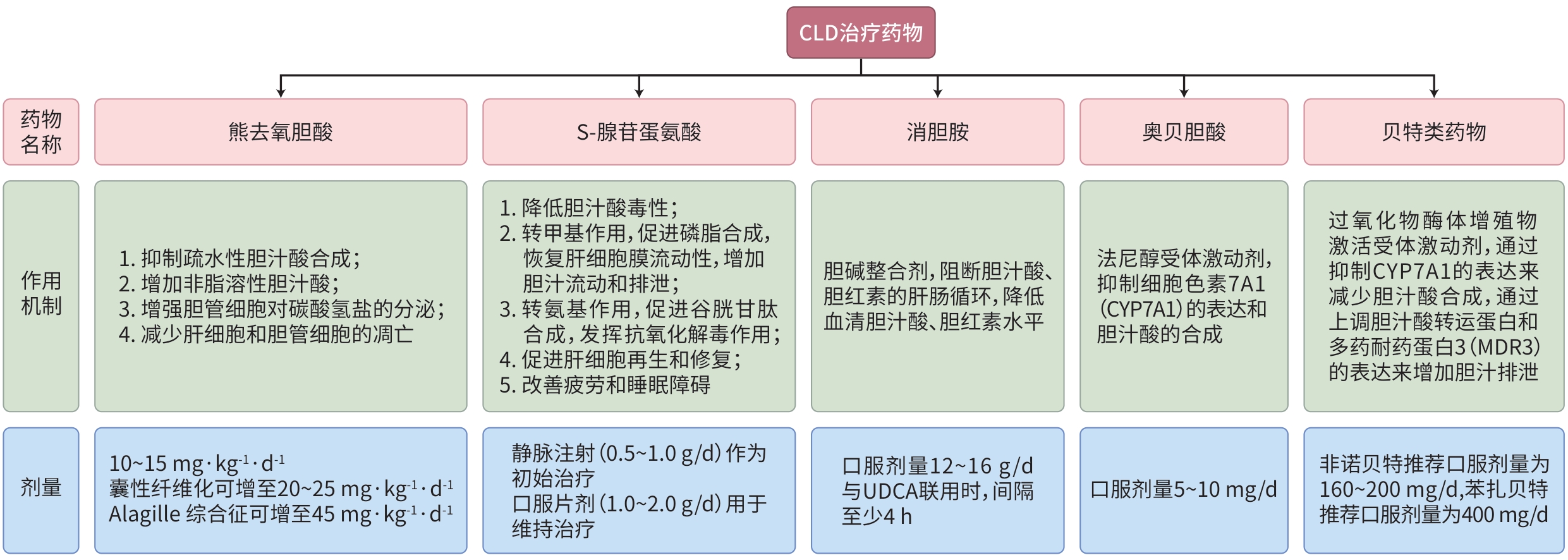
| [1] |
LUO X, LU LG. Progress in the management of patients with cholestatic liver disease: Where are we and where are we going?[J]. J Clin Transl Hepatol, 2024, 12( 6): 581- 588. DOI: 10.14218/JCTH.2023.00519. |
| [2] |
LU LG. Guidelines for the management of cholestatic liver diseases(2021)[J]. J Clin Transl Hepatol, 2022, 10( 4): 757- 769. DOI: 10.14218/jcth.2022.00147. |
| [3] |
CHAZOUILLERES O, BEUERS U, BERGQUIST A, et al. EASL Clinical Practice Guidelines on sclerosing cholangitis[J]. J Hepatol, 2022, 77( 3): 761- 806. DOI: 10.1016/j.jhep.2022.05.011. |
| [4] |
|
| [5] |
KARLSEN TH, FOLSERAAS T, THORBURN D, et al. Primary sclerosing cholangitis-a comprehensive review[J]. J Hepatol, 2017, 67( 6): 1298- 1323. DOI: 10.1016/j.jhep.2017.07.022. |
| [6] |
BOWLUS CL, ARRIVÉ L, BERGQUIST A, et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma[J]. Hepatology, 2023, 77( 2): 659- 702. DOI: 10.1002/hep.32771. |
| [7] |
MANNS MP, BERGQUIST A, KARLSEN TH, et al. Primary sclerosing cholangitis[J]. Nat Rev Dis Primers, 2025, 11: 17. DOI: 10.1038/s41572-025-00600-x. |
| [8] |
European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of cholestatic liver diseases[J]. J Hepatol, 2009, 51( 2): 237- 267. DOI: 10.1016/j.jhep.2009.04.009. |
| [9] |
HARMS MH, van BUUREN HR, CORPECHOT C, et al. Ursodeoxycholic acid therapy and liver transplant-free survival in patients with primary biliary cholangitis[J]. J Hepatol, 2019, 71( 2): 357- 365. DOI: 10.1016/j.jhep.2019.04.001. |
| [10] |
HIRSCHFIELD GM, DYSON JK, ALEXANDER GJM, et al. The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines[J]. Gut, 2018, 67( 9): 1568- 1594. DOI: 10.1136/gutjnl-2017-315259. |
| [11] |
European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis[J]. J Hepatol, 2017, 67( 1): 145- 172. DOI: 10.1016/j.jhep.2017.03.022. |
| [12] |
LINDOR KD, BOWLUS CL, BOYER J, et al. Primary biliary cholangitis: 2021 practice guidance update from the American Association for the Study of Liver Diseases[J]. Hepatology, 2022, 75( 4): 1012- 1013. DOI: 10.1002/hep.32117. |
| [13] |
HOSONUMA K, SATO K, YAMAZAKI Y, et al. A prospective randomized controlled study of long-term combination therapy using ursodeoxycholic acid and bezafibrate in patients with primary biliary cirrhosis and dyslipidemia[J]. Am J Gastroenterol, 2015, 110( 3): 423- 431. DOI: 10.1038/ajg.2015.20. |
| [14] |
HONDA A, TANAKA A, KANEKO T, et al. Bezafibrate improves GLOBE and UK-PBC scores and long-term outcomes in patients with primary biliary cholangitis[J]. Hepatology, 2019, 70( 6): 2035- 2046. DOI: 10.1002/hep.30552. |
| [15] |
TANAKA A, HIROHARA J, NAKANO T, et al. Association of bezafibrate with transplant-free survival in patients with primary biliary cholangitis[J]. J Hepatol, 2021, 75( 3): 565- 571. DOI: 10.1016/j.jhep.2021.04.010. |
| [16] |
van HOOFF MC, WERNER E, van der MEER AJ. Treatment in primary biliary cholangitis: Beyond ursodeoxycholic acid[J]. Eur J Intern Med, 2024, 124: 14- 21. DOI: 10.1016/j.ejim.2024.01.030. |
| [17] |
SORET PA, LAM L, CARRAT F, et al. Combination of fibrates with obeticholic acid is able to normalise biochemical liver tests in patients with difficult-to-treat primary biliary cholangitis[J]. Aliment Pharmacol Ther, 2021, 53( 10): 1138- 1146. DOI: 10.1111/apt.16336. |
| [18] |
ASSIS DN, BOWLUS CL. Recent advances in the management of primary sclerosing cholangitis[J]. Clin Gastroenterol Hepatol, 2023, 21( 8): 2065- 2075. DOI: 10.1016/j.cgh.2023.04.004. |
| [19] |
OLSSON R, BOBERG KM, de MUCKADELL OS, et al. High-dose ursodeoxycholic acid in primary sclerosing cholangitis: A 5-year multicenter, randomized, controlled study[J]. Gastroenterology, 2005, 129( 5): 1464- 1472. DOI: 10.1053/j.gastro.2005.08.017. |
| [20] |
IMAM MH, SINAKOS E, GOSSARD AA, et al. High-dose ursodeoxycholic acid increases risk of adverse outcomes in patients with early stage primary sclerosing cholangitis[J]. Aliment Pharmacol Ther, 2011, 34( 10): 1185- 1192. DOI: 10.1111/j.1365-2036.2011.04863.x. |
| [21] |
KOWDLEY KV, VUPPALANCHI R, LEVY C, et al. A randomized, placebo-controlled, phase II study of obeticholic acid for primary sclerosing cholangitis[J]. J Hepatol, 2020, 73( 1): 94- 101. DOI: 10.1016/j.jhep.2020.02.033. |
| [22] |
TABIBIAN JH, O’HARA SP, TRUSSONI CE, et al. Absence of the intestinal microbiota exacerbates hepatobiliary disease in a murine model of primary sclerosing cholangitis[J]. Hepatology, 2016, 63( 1): 185- 196. DOI: 10.1002/hep.27927. |
| [23] |
DAVIES YK, COX KM, ABDULLAH BA, et al. Long-term treatment of primary sclerosing cholangitis in children with oral vancomycin: An immunomodulating antibiotic[J]. J Pediatr Gastroenterol Nutr, 2008, 47( 1): 61- 67. DOI: 10.1097/MPG.0b013e31816fee95. |
| [24] |
DENEAU MR, MACK C, MOGUL D, et al. Oral vancomycin, ursodeoxycholic acid, or No therapy for pediatric primary sclerosing cholangitis: A matched analysis[J]. Hepatology, 2021, 73( 3): 1061- 1073. DOI: 10.1002/hep.31560. |
| [25] |
LAMMERS WJ, HIRSCHFIELD GM, CORPECHOT C, et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy[J]. Gastroenterology, 2015, 149( 7): 1804- 1812. e 4. DOI: 10.1053/j.gastro.2015.07.061. |
| [26] |
GOET JC, MURILLO PEREZ CF, HARMS MH, et al. A comparison of prognostic scores(mayo, UK-PBC, and GLOBE) in primary biliary cholangitis[J]. Am J Gastroenterol, 2021, 116( 7): 1514- 1522. DOI: 10.14309/ajg.0000000000001285. |
| [27] |
CARBONE M, SHARP SJ, FLACK S, et al. The UK-PBC risk scores: Derivation and validation of a scoring system for long-term prediction of end-stage liver disease in primary biliary cholangitis[J]. Hepatology, 2016, 63( 3): 930- 950. DOI: 10.1002/hep.28017. |
| [28] |
HARMS MH, de VEER RC, LAMMERS WJ, et al. Number needed to treat with ursodeoxycholic acid therapy to prevent liver transplantation or death in primary biliary cholangitis[J]. Gut, 2020, 69( 8): 1502- 1509. DOI: 10.1136/gutjnl-2019-319057. |
| [29] |
MURILLO PEREZ CF, HARMS MH, LINDOR KD, et al. Goals of treatment for improved survival in primary biliary cholangitis: Treatment target should be bilirubin within the normal range and normalization of alkaline phosphatase[J]. Am J Gastroenterol, 2020, 115( 7): 1066- 1074. DOI: 10.14309/ajg.0000000000000557. |
| [30] |
CORPECHOT C, LEMOINNE S, SORET PA, et al. Adequate versus deep response to ursodeoxycholic acid in primary biliary cholangitis: To what extent and under what conditions is normal alkaline phosphatase level associated with complication-free survival gain?[J]. Hepatology, 2024, 79( 1): 39- 48. DOI: 10.1097/HEP.0000000000000529. |
| [31] |
CHEUNG AC, LAMMERS WJ, MURILLO PEREZ CF, et al. Effects of age and sex of response to ursodeoxycholic acid and transplant-free survival in patients with primary biliary cholangitis[J]. Clin Gastroenterol Hepatol, 2019, 17( 10): 2076- 2084. e 2. DOI: 10.1016/j.cgh.2018.12.028. |
| [32] |
CORPECHOT C, CARRAT F, GAOUAR F, et al. Liver stiffness measurement by vibration-controlled transient elastography improves outcome prediction in primary biliary cholangitis[J]. J Hepatol, 2022, 77( 6): 1545- 1553. DOI: 10.1016/j.jhep.2022.06.017. |
| [33] |
CRISTOFERI L, CALVARUSO V, OVERI D, et al. Accuracy of transient elastography in assessing fibrosis at diagnosis in Naïve patients with primary biliary cholangitis: A dual cut-off approach[J]. Hepatology, 2021, 74( 3): 1496- 1508. DOI: 10.1002/hep.31810. |
| [34] |
KIM WR, THERNEAU TM, WIESNER RH, et al. A revised natural history model for primary sclerosing cholangitis[J]. Mayo Clin Proc, 2000, 75( 7): 688- 694. DOI: 10.4065/75.7.688. |
| [35] |
GOODE EC, CLARK AB, MELLS GF, et al. Factors associated with outcomes of patients with primary sclerosing cholangitis and development and validation of a risk scoring system[J]. Hepatology, 2019, 69( 5): 2120- 2135. DOI: 10.1002/hep.30479. |
| [36] |
de VRIES EM, WANG JF, WILLIAMSON KD, et al. A novel prognostic model for transplant-free survival in primary sclerosing cholangitis[J]. Gut, 2018, 67( 10): 1864- 1869. DOI: 10.1136/gutjnl-2016-313681. |
| [37] |
EATON JE, VESTERHUS M, MCCAULEY BM, et al. Primary sclerosing cholangitis risk estimate tool(PREsTo) predicts outcomes of the disease: A derivation and validation study using machine learning[J]. Hepatology, 2020, 71( 1): 214- 224. DOI: 10.1002/hep.30085. |
| [38] |
DENEAU MR, MACK C, PERITO ER, et al. The sclerosing cholangitis outcomes in pediatrics(SCOPE) index: A prognostic tool for children[J]. Hepatology, 2021, 73( 3): 1074- 1087. DOI: 10.1002/hep.31393. |



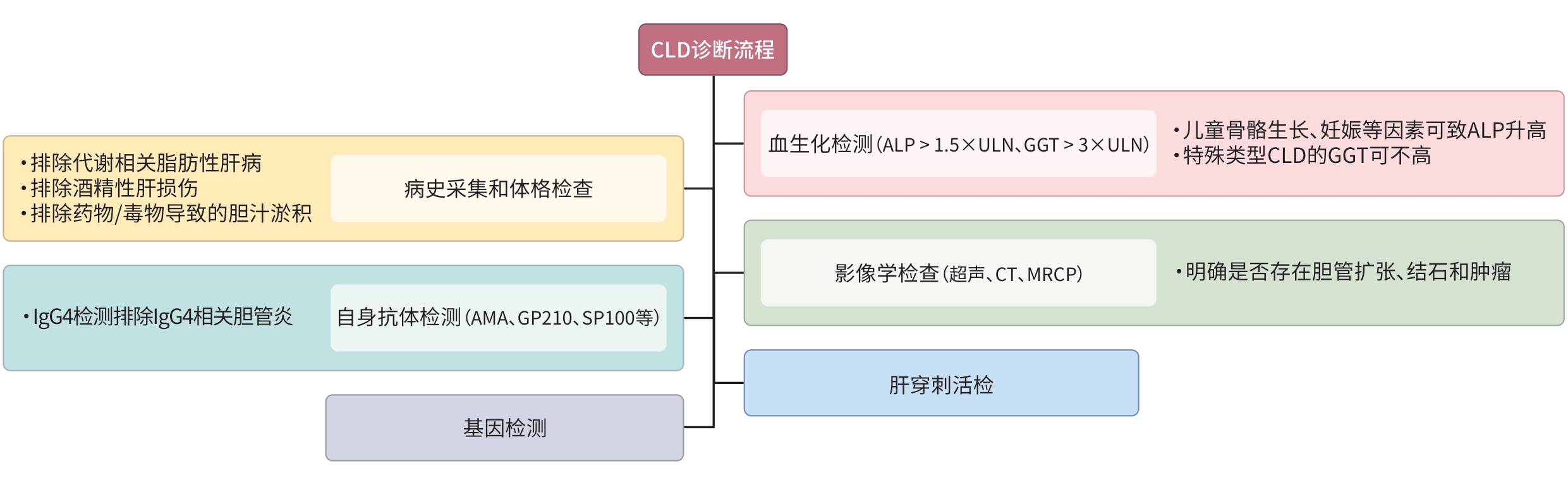




 DownLoad:
DownLoad: