| [1] |
LI J, ZHENG KY, ZHANG BB. Mechanism of action of bile acid metabolism in regulating cholestatic liver disease and the research and development of drugs[J]. J Clin Hepatol, 2021, 37( 10): 2482- 2487. DOI: 10.3969/j.issn.1001-5256.2021.10.048. |
| [2] |
YANG ZH, DANZENG AW, LIU QM, et al. The role of nuclear receptors in the pathogenesis and treatment of non-alcoholic fatty liver disease[J]. Int J Biol Sci, 2024, 20( 1): 113- 126. DOI: 10.7150/ijbs.87305. |
| [3] |
SHEN H, HU M, WEI ZH, et al. Bile formation, secretion, and excretion and the pathogenesis of cholestasis[J]. J Clin Hepatol, 2019, 35( 2): 431- 437. DOI: 10.3969/j.issn.1001-5256.2019.02.043. |
| [4] |
LU LG. Attach importance to clinical management of cholestatic liver disease[J]. J Intern Med Concepts Pract, 2022, 17( 1): 1- 3. DOI: 10.16138/j.1673-6087.2022.01.001. |
| [5] |
IIDA M, HIGASHIDE A, OHTOMO S, et al. Association of bile acids composition and synthetic pathway with therapeutic effect of bezafibrate in chronic cholestatic liver disease[J]. J Hepatol, 2022, 77: S600. DOI: 10.1016/S0168-8278(22)01517-3. |
| [6] |
HEIMERL S, BOETTCHER A, KAUL H, et al. Lipid profiling of lipoprotein X: Implications for dyslipidemia in cholestasis[J]. Biochim Biophys Acta, 2016, 1861( 8 Pt A): 681- 687. DOI: 10.1016/j.bbalip.2016.04.016. |
| [7] |
BURHAN RAOOF I, ABDALAH ME, ABDULMAHDI MOHSIN R. Relation between vit. D3 and other metabolic risk factors in patients with cholestatic liver disease[J]. Res J Pharm Technol, 2022: 3086- 3090. DOI: 10.52711/0974-360x.2022.00516. |
| [8] |
CHROSTEK L, BAUER A, GRUSZEWSKA E, et al. T273 Serum transferrin isoforms in autoimmune cholestatic liver diseases[J]. Clin Chim Acta, 2022, 530: S192. DOI: 10.1016/j.cca.2022.04.518. |
| [9] |
SHNEIDER BL, KAMATH BM, MAGEE JC, et al. Use of funded multicenter prospective longitudinal databases to inform clinical trials in rare diseases-Examination of cholestatic liver disease in Alagille syndrome[J]. Hepatol Commun, 2022, 6( 8): 1910- 1921. DOI: 10.1002/hep4.1970. |
| [10] |
PUGLIESE N, ARCARI I, AGHEMO A, et al. Osteosarcopenia in autoimmune cholestatic liver diseases: Causes, management, and challenges[J]. World J Gastroenterol, 2022, 28( 14): 1430- 1443. DOI: 10.3748/wjg.v28.i14.1430. |
| [11] |
HUYGEN LPM, WESTERINK J, MOL GC, et al. When LDL cholesterol is not LDL cholesterol: LpX, A clinical lesson[J]. JACC Case Rep, 2022, 4( 11): 690- 693. DOI: 10.1016/j.jaccas.2022.03.009. |
| [12] |
DUENGELHOEF P, HARTL J, RÜTHER D, et al. SARS-CoV-2 vaccination response in patients with autoimmune hepatitis and autoimmune cholestatic liver disease[J]. United European Gastroenterol J, 2022, 10( 3): 319- 329. DOI: 10.1002/ueg2.12218. |
| [13] |
IBRAHIM SH, KAMATH BM, LOOMES KM, et al. Cholestatic liver diseases of genetic etiology: Advances and controversies[J]. Hepatology, 2022, 75( 6): 1627- 1646. DOI: 10.1002/hep.32437. |
| [14] |
KATTAH L, GÓMEZ A, GUTIÉRREZ S, et al. Hypercholesterolemia due to lipoprotein X: Case report and thematic review[J]. Clin Med Insights Endocrinol Diabetes, 2019, 12: 1179551419878687. DOI: 10.1177/1179551419878687. |
| [15] |
ALBARGAWI M, ABDULAAL I. Significant high lipid profile in a woman with obstructive jaundice[J]. JCEM Case Rep, 2023, 1( 4): luad080. DOI: 10.1210/jcemcr/luad080. |
| [16] |
FELKER TE, HAMILTON RL, HAVEL RJ. Secretion of lipoprotein-X by perfused livers of rats with cholestasis[J]. Proc Natl Acad Sci USA, 1978, 75( 7): 3459- 3463. DOI: 10.1073/pnas.75.7.3459. |
| [17] |
CRAWFORD AR, SMITH AJ, HATCH VC, et al. Hepatic secretion of phospholipid vesicles in the mouse critically depends on mdr2 or MDR3 P-glycoprotein expression. Visualization by electron microscopy[J]. J Clin Invest, 1997, 100( 10): 2562- 2567. DOI: 10.1172/JCI119799. |
| [18] |
LI M, PING J, XU LM. Establishment of a primary liver cancer model in Mdr2 gene knockout mice: An observational study[J]. Acta Lab Animalis Sci Sin, 2022, 30( 8): 1058- 1063. DOI: 10.3969/j.issn.1005-4847.2022.08.006. |
| [19] |
ELFERINK RP, OTTENHOFF R, van MARLE J, et al. Class III P-glycoproteins mediate the formation of lipoprotein X in the mouse[J]. J Clin Invest, 1998, 102( 9): 1749- 1757. DOI: 10.1172/JCI3597. |
| [20] |
NUÑO-LÁMBARRI N, BARBERO-BECERRA VJ, URIBE M, et al. Elevated cholesterol levels have a poor prognosis in a cholestasis scenario[J]. J Biochem Mol Toxicol, 2017, 31( 1): 1- 6. DOI: 10.1002/jbt.21831. |
| [21] |
KLOOSTERMAN E, DIJKSTRA T, VERKADE HJ. 3.9 nutritional management in cholestatic liver disease[J]. World Rev Nutr Diet, 2022, 124: 277- 284. DOI: 10.1159/000516985. |
| [22] |
RESHETNYAK VI, MAEV IV. Features of lipid metabolism disorders in primary biliary cholangitis[J]. Biomedicines, 2022, 10( 12): 3046. DOI: 10.3390/biomedicines10123046. |
| [23] |
FELLIN R, MANZATO E. Lipoprotein-X fifty years after its original discovery[J]. Nutr Metab Cardiovasc Dis, 2019, 29( 1): 4- 8. DOI: 10.1016/j.numecd.2018.09.006. |
| [24] |
AHMED W, JEYARAJ R, REFFITT D, et al. Nasobiliary drainage: An effective treatment for pruritus in cholestatic liver disease[J]. Frontline Gastroenterol, 2022, 13( 5): 416- 422. DOI: 10.1136/flgastro-2021-102025. |
| [25] |
CHAN WK, LAW EY, LING TK, et al. Lipoprotein-X hyperlipidaemia in Chinese paediatric patients with liver graft-versus-host disease post-haematopoietic stem cell transplantation: Two case reports[J]. Hong Kong Med J, 2023, 29( 1): 76- 78. DOI: 10.12809/hkmj219765. |
| [26] |
HEINL RE, TENNANT HM, RICKETTS JC, et al. Lipoprotein-X disease in the setting of severe cholestatic hepatobiliary autoimmune disease[J]. J Clin Lipidol, 2017, 11( 1): 282- 286. DOI: 10.1016/j.jacl.2016.09.016. |
| [27] |
EDWARDS CM, OTAL MP, STACPOOLE PW. Lipoprotein-X fails to inhibit hydroxymethylglutaryl coenzyme A reductase in HepG2 cells[J]. Metabolism, 1993, 42( 7): 807- 813. DOI: 10.1016/0026-0495(93)90051-o. |
| [28] |
|
| [29] |
LOAEZA-DEL CASTILLO AM, GAYTÁN-SANTILLÁN A, LÓPEZ-TELLO A, et al. Patterns of serum lipids derangements and cardiovascular risk assessment in patients with primary biliary cholangitis[J]. Ann Hepatol, 2019, 18( 6): 879- 882. DOI: 10.1016/j.aohep.2019.07.006. |
| [30] |
MIYAHARA K, KASAHARA N, KONDO Y, et al. Changes in plasma lipids and abnormal lipoproteins in a patient with drug-induced cholestatic hepatitis[J]. Jpn J Med, 1991, 30( 4): 354- 359. DOI: 10.2169/internalmedicine1962.30.354. |
| [31] |
PATSCH JR, AUNE KC, GOTTO AM Jr, et al. Isolation, chemical characterization, and biophysical properties of three different abnormal lipoproteins: LP-X1, LP-X2, and LP-X3[J]. J Biol Chem, 1977, 252( 6): 2113- 2120.
|
| [32] |
HERZUM I, GIEHL C, SOUFI M, et al. Interference in a homogeneous assay for low-density lipoprotein cholesterol by lipoprotein X[J]. Clin Chem Lab Med, 2007, 45( 5): 667- 671. DOI: 10.1515/CCLM.2007.114. |
| [33] |
ĆWIKLIŃSKA A, MICKIEWICZ A, KOWALSKI R, et al. Detection of lipoprotein X(LpX): A challenge in patients with severe hypercholesterolaemia[J]. J Med Biochem, 2020, 39( 3): 283- 289. DOI: 10.2478/jomb-2019-0038. |
| [34] |
FREEMAN LA, SHAMBUREK RD, SAMPSON ML, et al. Plasma lipoprotein-X quantification on filipin-stained gels: Monitoring recombinant LCAT treatment ex vivo[J]. J Lipid Res, 2019, 60( 5): 1050- 1057. DOI: 10.1194/jlr.D090233. |
| [35] |
WOLF PL. Clinical significance of serum high-molecular-mass alkaline phosphatase, alkaline phosphatase-lipoprotein-X complex, and intestinal variant alkaline phosphatase[J]. J Clin Lab Anal, 1994, 8( 3): 172- 176. DOI: 10.1002/jcla.1860080311. |
| [36] |
FOLEY KF, SILVEIRA MG, HORNSETH JM, et al. A patient with primary biliary cirrhosis and elevated LDL cholesterol[J]. Clin Chem, 2009, 55( 1): 187- 191; disscusion 191- 192. DOI: 10.1373/clinchem.2008.108720. |
| [37] |
SUZUKI L, HIRAYAMA S, FUKUI M, et al. Lipoprotein-X in cholestatic patients causes xanthomas and promotes foam cell formation in human macrophages[J]. J Clin Lipidol, 2017, 11( 1): 110- 118. DOI: 10.1016/j.jacl.2016.10.013. |
| [38] |
PETRIE E, HOPPMANN NA, WILCOX CM, et al. Gastric xanthomatosis secondary to lipoprotein X in primary biliary cholangitis[J]. J Investig Med High Impact Case Rep, 2022, 10: 23247096221089488. DOI: 10.1177/23247096221089488. |
| [39] |
COLANTUONO R, PAVANELLO C, PIETROBATTISTA A, et al. Case report: Unusual and extremely severe lipoprotein X-mediated hypercholesterolemia in extrahepatic pediatric cholestasis[J]. Front Pediatr, 2022, 10: 969081. DOI: 10.3389/fped.2022.969081. |
| [40] |
CUPERUS FJC, HALILBASIC E, TRAUNER M. Fibrate treatment for primary biliary cirrhosis[J]. Curr Opin Gastroenterol, 2014, 30( 3): 279- 286. DOI: 10.1097/MOG.0000000000000056. |
| [41] |
JESWANI BM, SHARMA S, RATHORE SS, et al. PCSK9 inhibitors: The evolving future[J]. Health Sci Rep, 2024, 7( 11): e70174. DOI: 10.1002/hsr2.70174. |
| [42] |
SEQUEIRA C, COELHO M, COSTA SANTOS I, et al. Severe hypercholesterolemia mediated by lipoprotein X in an immunosuppressed patient: A case report[J]. GE Port J Gastroenterol, 2022, 30( 5): 398- 402. DOI: 10.1159/000526854. |
| [43] |
AMAR MJA, FREEMAN LA, NISHIDA T, et al. LCAT protects against Lipoprotein-X formation in a murine model of drug-induced intrahepatic cholestasis[J]. Pharmacol Res Perspect, 2019, 8( 1): e00554. DOI: 10.1002/prp2.554. |
| [44] |
ZHAO YF, LI Y, WANG F, et al. CES1-triggered liver-specific cargo release of CRISPR/Cas9 elements by cationic triadic copolymeric nanoparticles targeting gene editing of PCSK9 for hyperlipidemia amelioration[J]. Adv Sci(Weinh), 2023, 10( 19): e2300502. DOI: 10.1002/advs.202300502. |



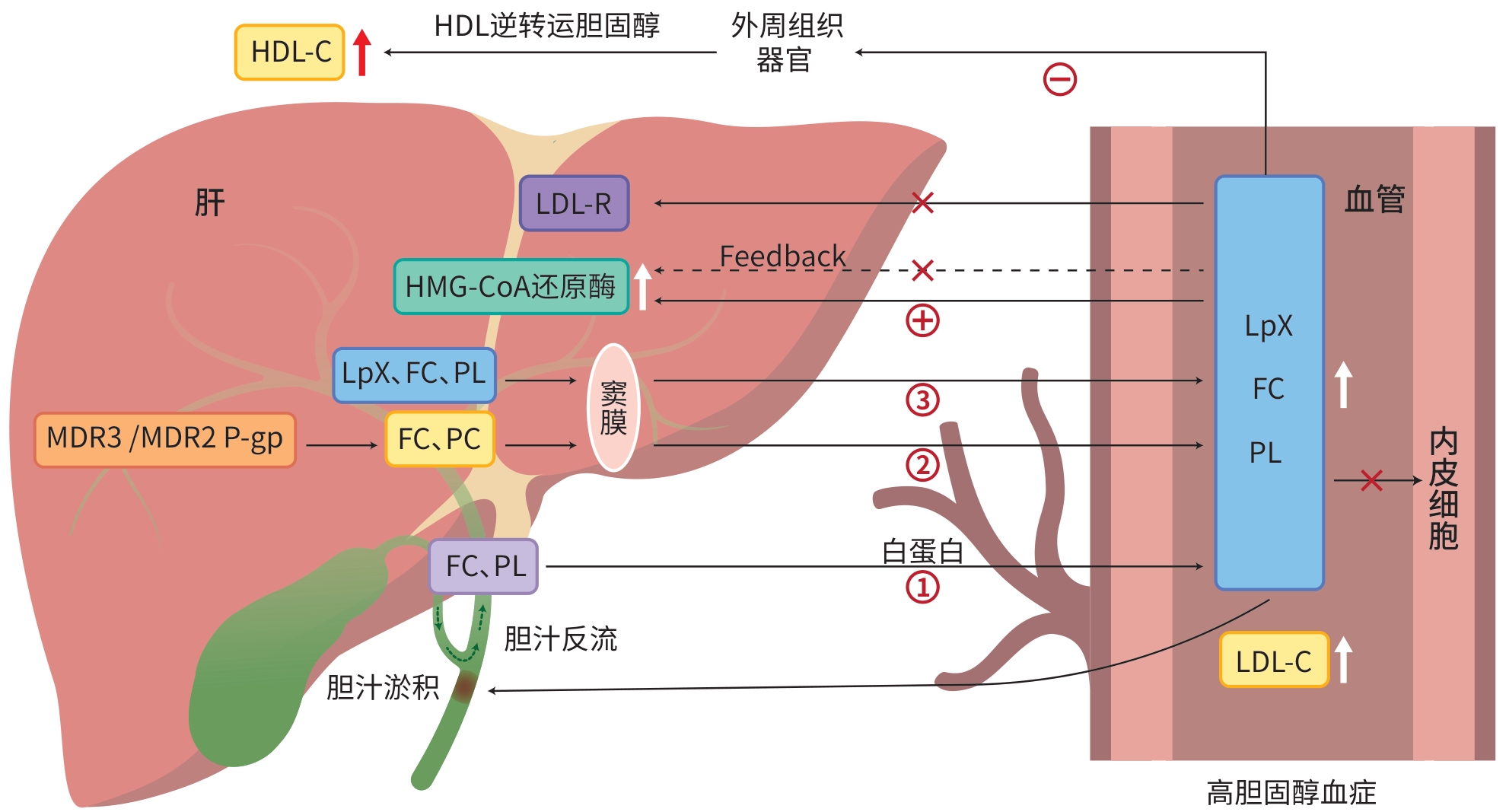




 DownLoad:
DownLoad: