氨氯地平及左氨氯地平对大鼠体内仑伐替尼药物动力学的影响及其机制
DOI: 10.12449/JCH241118
Effect of amlodipine and levamlodipine on the pharmacokinetics of lenvatinib in rats and related mechanisms
-
摘要:
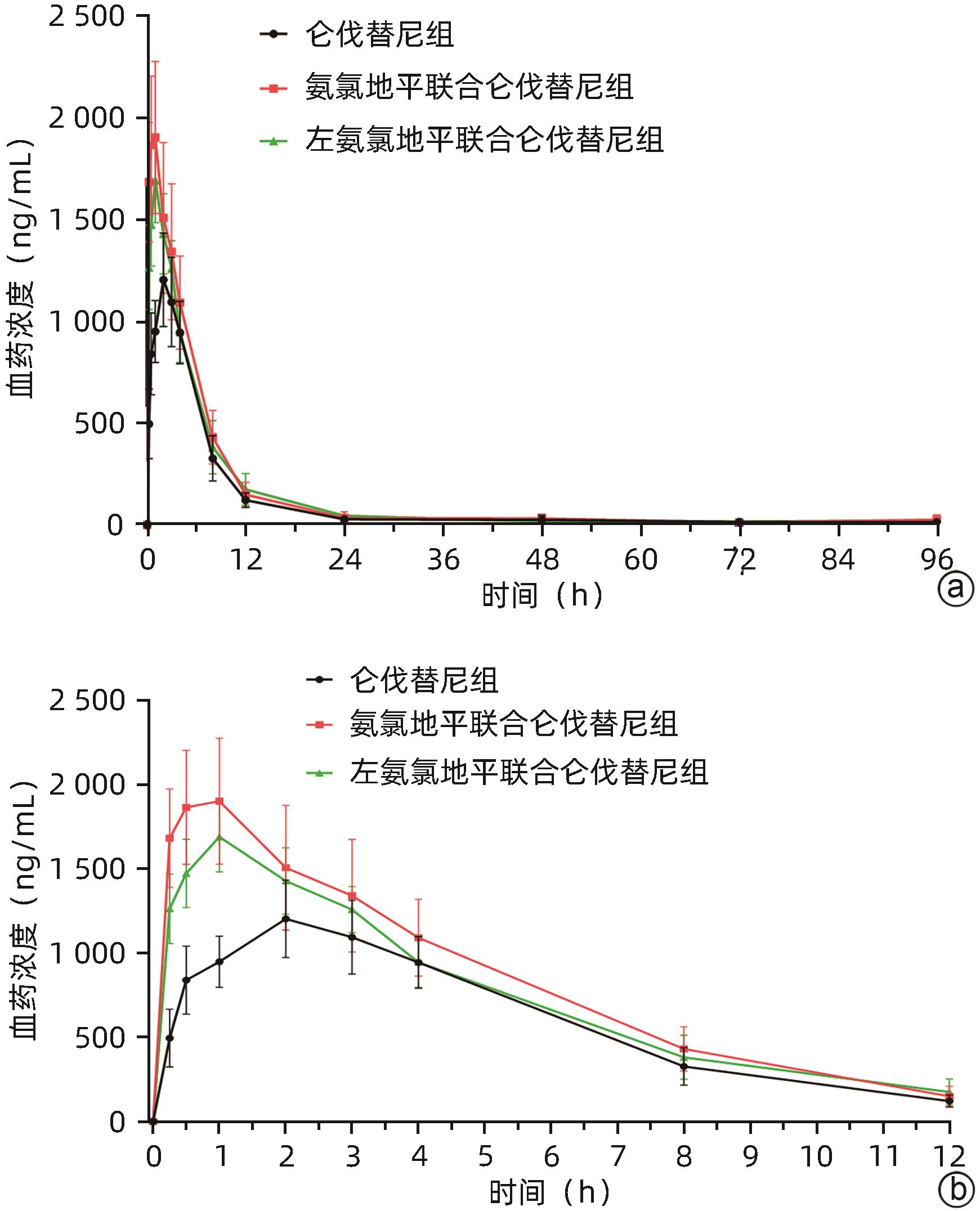
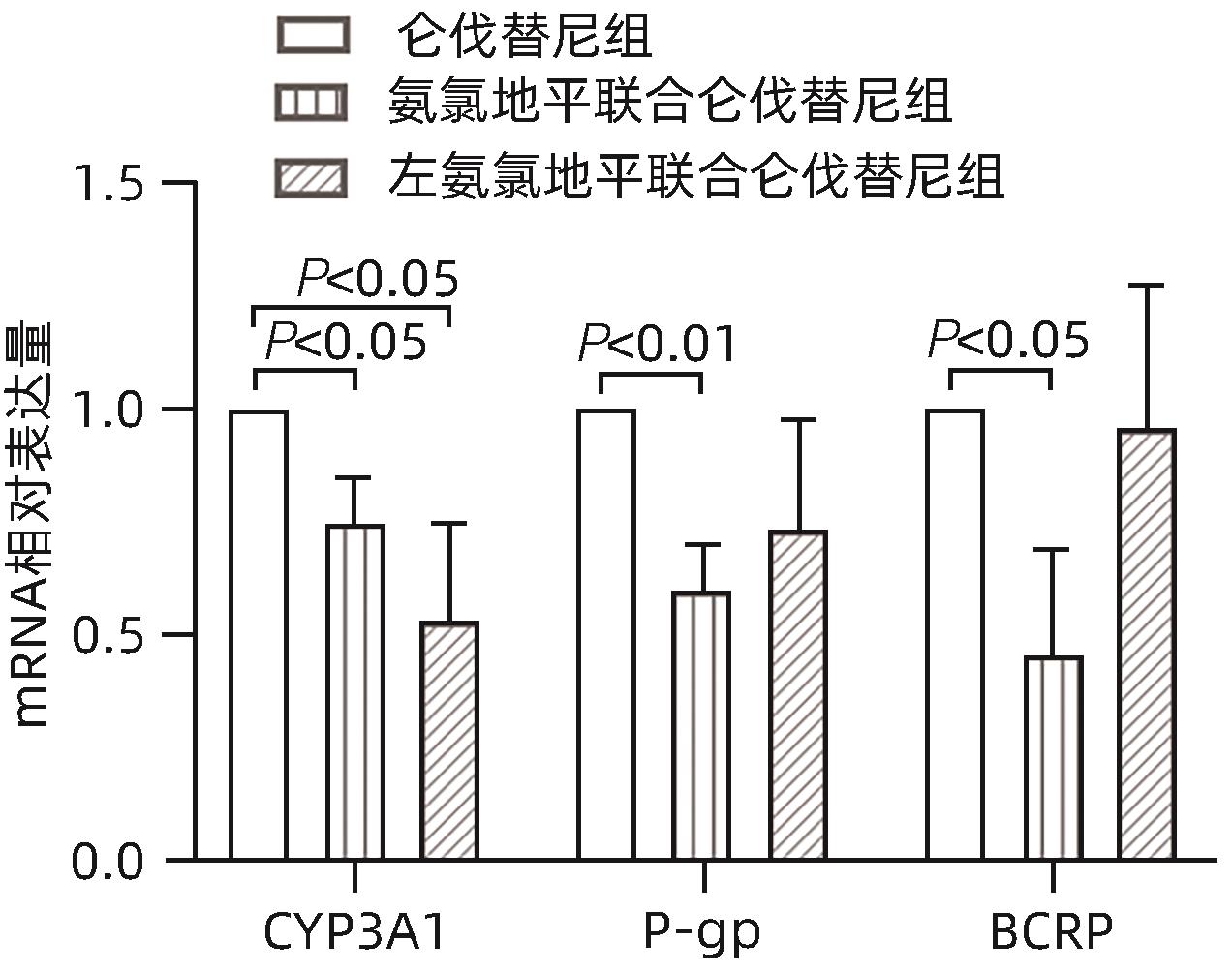
目的 研究氨氯地平及左氨氯地平对仑伐替尼药物动力学的影响并探究相关机制。 方法 选取18只雄性SD大鼠随机分为3组,包括仑伐替尼(1.2 mg/kg)组、氨氯地平(1.0 mg/kg)联合仑伐替尼组和左氨氯地平(0.5 mg/kg)联合仑伐替尼组,每组各6只。分别用0.5%羧甲基纤维素钠、氨氯地平及左氨氯地平灌胃液预处理8 d,末次灌胃后给予仑伐替尼,并按照规定的采血时间点眼内眦静脉丛采血。采用超高效液相色谱串联质谱(UPLC-MS/MS)法测定大鼠血浆中仑伐替尼的药物浓度,非房室模型计算药物动力学参数。采用RT-qPCR检测大鼠肝组织中细胞色素P450 3A1(CYP3A1)、P-糖蛋白(P-gp)和乳腺癌耐药蛋白(BCRP)mRNA表达。符合正态分布的计量资料多组间比较采用单因素方差分析,进一步两两比较采用Dunnett-t检验;不符合正态分布的计量资料多组间比较采用Kruskal-Wallis H检验。 结果 3组间药时曲线下面积AUC0-∞(F=4.567,P<0.05)、清除率CLz/F(F=5.038,P<0.05)和峰浓度Cmax(F=11.667,P<0.01)比较差异均存在统计学意义(P值均<0.05),与仑伐替尼组比较,氨氯地平联合仑伐替尼组AUC0-∞升高36.1%(P<0.05)、CLz/F下降26.1%(P<0.05)、Cmax升高56.7%(P<0.01),左氨氯地平联合仑伐替尼组Cmax升高37.7%(P<0.05);RT-qPCR结果显示,3组间CYP3A1、P-gp和BCRP mRNA的表达差异均有统计学意义(F值分别为10.160、5.350、5.237,P值均<0.05),与仑伐替尼组比较,氨氯地平联合仑伐替尼组大鼠肝脏CYP3A1、P-gp和BCRP mRNA表达水平明显下降(P值均<0.05),而左氨氯地平联合仑伐替尼组大鼠肝脏中CYP3A1 mRNA表达水平也明显下降(P<0.05)。 结论 氨氯地平可以增加仑伐替尼的体内暴露量,作用机制可能与抑制肝脏中CYP3A1、P-gp和BCRP的mRNA表达有关;而左氨氯地平仅增加仑伐替尼的峰浓度。 -
关键词:
- 癌, 肝细胞 /
- 大鼠, Sprague-Dawley /
- 氨氯地平 /
- 仑伐替尼
Abstract:Objective To investigate the effect of amlodipine and levamlodipine on the pharmacokinetics of lenvatinib and related mechanisms. Methods A total of 18 male Sprague-Dawley rats were randomly divided into lenvatinib (1.2 mg/kg) group, amlodipine (1.0 mg/kg)+lenvatinib group, and levamlodipine (0.5 mg/kg)+lenvatinib group, with 6 rats in each group. The rats were pretreated with 0.5% sodium carboxymethyl cellulose, amlodipine or levamlodipine by gavage for 8 days, and lenvatinib was given after the last intragastric administration. Blood samples were collected from the intraocular canthus venous plexus at the specified time points. Ultra-performance liquid chromatography-tandem mass spectrometry was used to measure the plasma concentration of lenvatinib in rats, and a non-compartment model was used to calculate pharmacokinetic parameters. RT-qPCR was used to measure the mRNA expression levels of cytochrome P450 3A1 (CYP3A1), P-glycoprotein (P-gp), and breast cancer resistance protein (BCRP) in rat liver tissue. A one-way analysis of variance was used for comparison of normally distributed continuous data between multiple groups, and the Dunnett-t test was used for further comparison between two groups; the Kruskal-Wallis H test was used for comparison of non-normally distributed continuous data between groups. Results There were significant differences between the three groups in the area under the concentration-time curve AUC0-∞ (F=4.567, P<0.05), clearance rate CLz/F (F=5.038, P<0.05), and peak concentration Cmax (F=11.667, P<0.01). Compared with the lenvatinib group, the amlodipine+lenvatinib group had an increase in AUC0-∞ by 36.1% (P<0.05), a reduction in CLz/F by 26.1% (P<0.05), and an increase in Cmax by 56.7% (P<0.01), and the levamlodipine+lenvatinib group had an increase in Cmax by 37.7% (P<0.05). RT-qPCR showed that there were significant differences in the mRNA expression levels of CYP3A1, P-gp, and BCRP between the three groups (F=10.160, 5.350, and 5.237, all P<0.05), and compared with the lenvatinib group, the amlodipine+lenvatinib group had significant reductions in the mRNA expression levels of CYP3A1, P-gp, and BCRP in rat liver tissue (all P<0.05), while the levamlodipine+lenvatinib group had a significant reduction in the mRNA expression level of CYP3A1 in rat liver tissue (P<0.05). Conclusion Amlodipine can increase the in vivo exposure of lenvatinib possibly by inhibiting the mRNA expression of CYP3A1, P-gp, and BCRP in the liver, while levamlodipine only increases the peak concentration of lenvatinib. -
Key words:
- Carcinoma, Hepatocellular /
- Rats, Sprague-Dawley /
- Amlodipin /
- Lenvatinib
-
表 1 引物序列
Table 1. Primer sequences
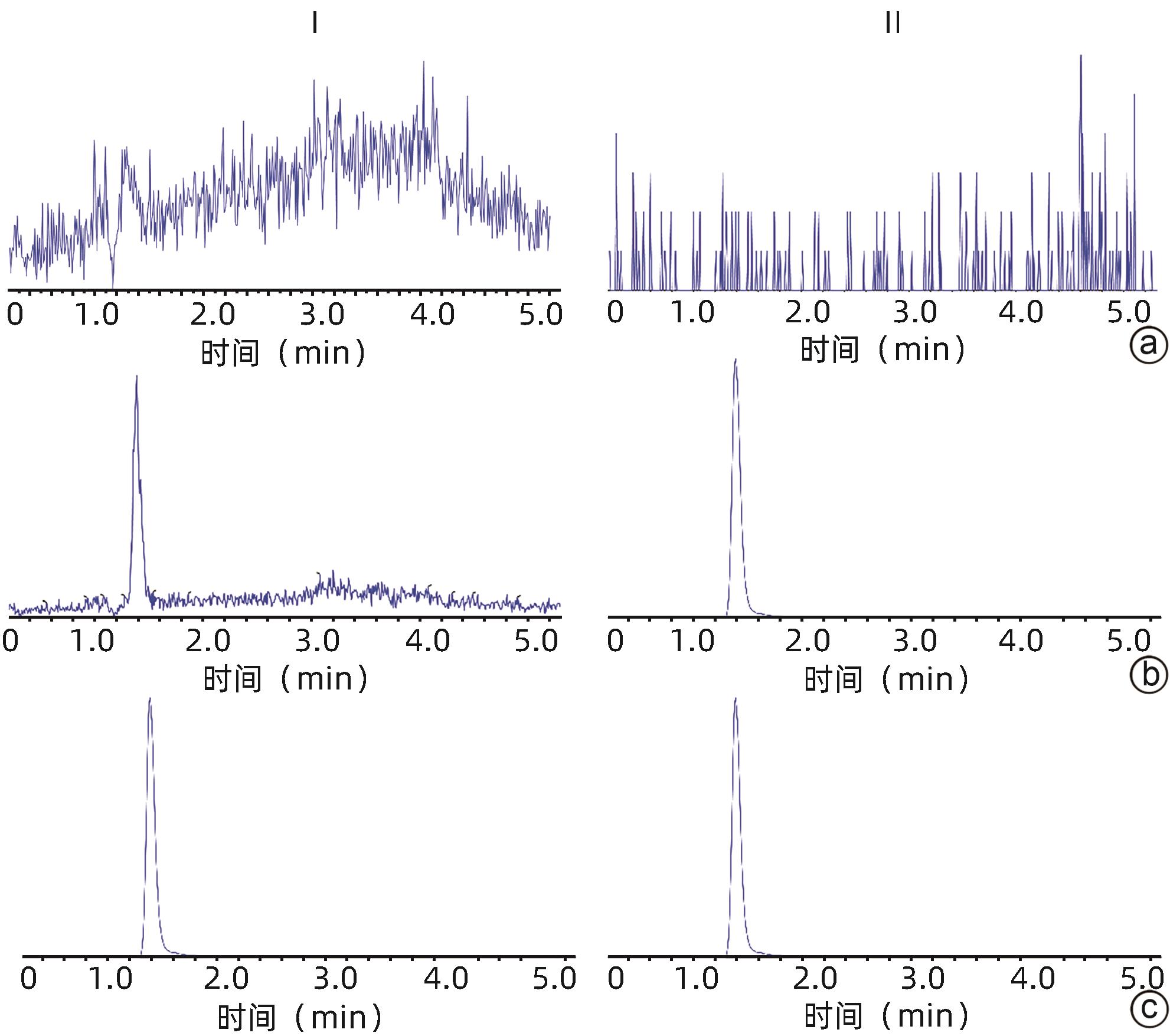
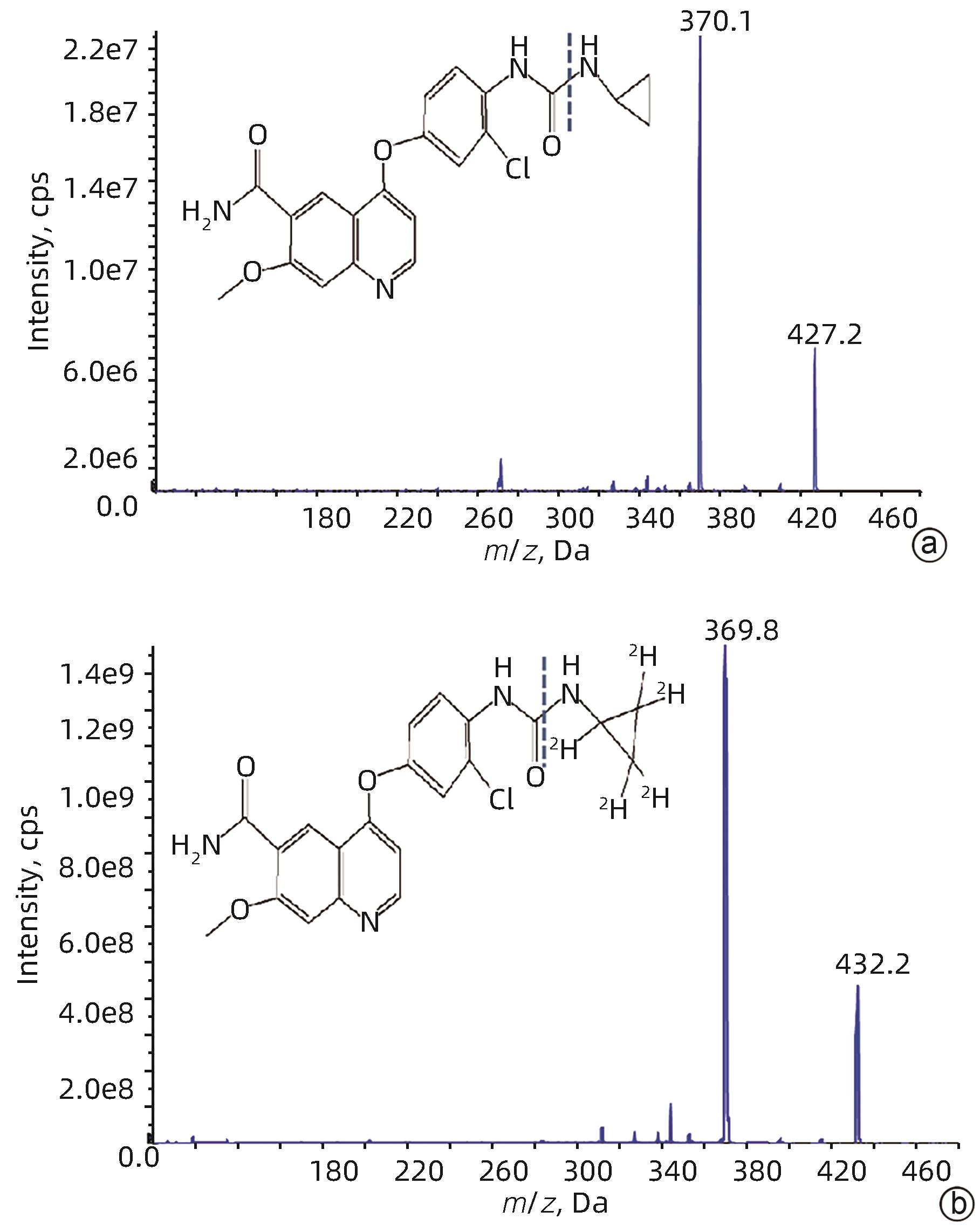
基因 正向引物序列 反向引物序列 GADPH 5'-GCCTTCCGTGTTCCTACC-3' 5'-GCCTGCTTCACCACCTTC-3' P-gp 5'-TCTGGTATGGGACTTCCTTGGT-3' 5'-TCCTTGTATGTTGTCGGGTTTG-3' BCRP 5'-TGAAGAGTGGCTTTCTAGTCCG-3' 5'-TTGAAATTGGCAGGTTGAGGTG-3' CYP3A1 5'-TGCATTGGCATGAGGTTTGC-3' 5'-TTCAGCAGAACTCCTTGAGGG-3' 表 2 大鼠血浆中仑伐替尼的精密度与准确度
Table 2. Precision and accuracy of LEN in rat plasma
理论质量浓度 批内(n=6) 批间(n=18) 实测质量浓度(ng/mL) RSD(%) RE(%) 实测质量浓度(ng/mL) RSD(%) RE(%) 2 ng/mL 2.09±0.12 5.6 4.5 2.05±0.09 4.6 2.7 5 ng/mL 5.47±0.15 2.7 9.0 5.45±0.14 2.5 9.0 100 ng/mL 102.90±3.56 3.5 2.9 103.22±4.38 4.2 3.2 2 000 ng/mL 2 068.33±126.56 6.1 3.4 2 067.78±107.73 5.2 3.4 表 3 大鼠血浆中仑伐替尼的提取回收率和基质效应
Table 3. Extraction recovery and matrix effect of LEN in rat plasma
理论质量浓度 提取回收率(%) RSD(%) 基质效应(%) RSD(%) 2 ng/mL 88.25±5.49 6.2 100.00±12.6 12.6 100 ng/mL 97.84±4.48 4.6 98.32±4.54 4.6 2 000 ng/mL 97.91±3.82 3.9 104.23±4.00 3.7 表 4 在不同条件下大鼠血浆中仑伐替尼的稳定性
Table 4. Stability of LEN in rat plasma under various conditions
条件 理论质量
浓度(ng/mL)
实测质量
浓度(ng/mL)
精密度
RSD(%)
准确度
RE(%)
室温8 h 5 5.47±0.11 2.0 9.4 100 103.85±6.58 6.3 3.9 2 000 2 053.33±107.83 5.3 2.7 进样器中12 h 5 5.40±0.16 2.9 8.1 100 102.75±3.22 3.1 2.8 2 000 2 081.67±106.47 5.1 4.1 -20 ℃冻融3次 5 5.42±0.16 2.9 8.3 100 105.83±3.76 3.6 5.8 2 000 1 945.00±106.16 5.5 -2.8 -20 ℃ 30 d 5 5.31±0.11 2.0 6.2 100 102.58±6.39 6.2 2.6 2 000 1 928.33±51.15 2.7 -3.6 表 5 单独使用及联合氨氯地平或左氨氯地平时仑伐替尼的药代动力学参数
Table 5. Pharmacokinetic parameters of lenvatinib alone andcombined with AML or LAML
参数 仑伐替尼组
(n=6)
仑伐替尼联合
氨氯地平组(n=6)
仑伐替尼联合
左氨氯地平组(n=6)
统计值 P值 AUC0-t (μg/L·h) 9 807.06±1 390.15 13 416.18±3 350.05 11 963.61±2 347.96 F=3.393 0.061 AUC0-∞(μg/L·h) 11 142.51±2 246.41 15 160.55±3 308.921) 12 456.64±2 788.95 F=4.567 0.028 t1/2(h) 55.22±37.64 74.10±50.40 35.01±13.90 F=2.317 0.133 Tmax(h) 1.50(0.50~3.00) 1.00(0.50~3.00) 1.00(1.00~2.00) H=2.686 0.261 Vz/F(L/kg) 8.18±4.81 8.63±6.00 4.96±1.89 F=1.065 0.369 CLz/F(L·h-1·kg-1) 0.11±0.02 0.08±0.021) 0.10±0.02 F=5.038 0.021 Cmax(μg/L) 1 238.33±164.25 1 940.00±344.272) 1 705.00±197.051) F=11.667 0.001 注:AUC0-t,0到最后一个采血点的药物浓度曲线下面积;AUC0-∞,时间从0到无穷大的药物浓度曲线下面积;t1/2,药物体内消除半衰期;Tmax,达峰时间;Vz/F,表观分布容积;CLz/F,清除率;Cmax,药峰浓度。与仑伐替尼组比较,1)P<0.05, 2)P<0.01。
-
[1] LI JJ, YANG HH, HUO G. Analysis of clinical features,cell morphology and prognostic factors in patients with primary liver cancer[J]. J Clin Exp Med, 2024, 23( 6): 566- 570. DOI: 10.3969/j.issn.1671-4695.2024.06.002.李姣姣, 杨会会, 霍刚. 原发性肝癌患者临床特征、细胞形态学分析及其预后的影响因素分析[J]. 临床和实验医学杂志, 2024, 23( 6): 566- 570. DOI: 10.3969/j.issn.1671-4695.2024.06.002. [2] General Office of National Health Commission. Standard for diagnosis and treatment of primary liver cancer(2022 edition)[J]. J Clin Hepatol, 2022, 38( 2): 288- 303. DOI: 10.3969/j.issn.1001-5256.2022.02.009.国家卫生健康委办公厅. 原发性肝癌诊疗指南(2022年版)[J]. 临床肝胆病杂志, 2022, 38( 2): 288- 303. DOI: 10.3969/j.issn.1001-5256.2022.02.009. [3] LI J, ZHANG YJ, XIA JL. Interpretation of NCCN clinical practice guidelines for hepatocellular carcinoma, version 1.2023[J]. J Pract Oncol, 2023, 38( 5): 408- 415. DOI: 10.13267/j.cnki.syzlzz.2023.064.李婕, 章赟杰, 夏景林. 2023年第1版NCCN肝细胞癌临床实践指南更新解读[J]. 实用肿瘤杂志, 2023, 38( 5): 408- 415. DOI: 10.13267/j.cnki.syzlzz.2023.064. [4] XU HC, WANG FL, XIE LH. Current status and perspectives in clinical treatment of intermediate and advanced primary hepatocellular carcinoma[J]. J Changchun Univ Chin Med, 2024, 40( 1): 103- 107. DOI: 10.13463/j.cnki.cczyy.2024.01.024.许华晨, 王凤玲, 谢林虎. 中晚期原发性肝细胞癌的临床治疗现状与展望[J]. 长春中医药大学学报, 2024, 40( 1): 103- 107. DOI: 10.13463/j.cnki.cczyy.2024.01.024. [5] AL-SALAMA ZT, SYED YY, SCOTT LJ. Lenvatinib: A review in hepatocellular carcinoma[J]. Drugs, 2019, 79( 6): 665- 674. DOI: 10.1007/s40265-019-01116-x. [6] ZHAO Y, ZHANG YN, WANG KT, et al. Lenvatinib for hepatocellular carcinoma: From preclinical mechanisms to anti-cancer therapy[J]. Biochim Biophys Acta Rev Cancer, 2020, 1874( 1): 188391. DOI: 10.1016/j.bbcan.2020.188391. [7] LI JM, WANG XQ, NING C, et al. Influences of ABC transporter and CYP3A4/5 genetic polymorphisms on the pharmacokinetics of lenvatinib in Chinese healthy subjects[J]. Eur J Clin Pharmacol, 2020, 76( 8): 1125- 1133. DOI: 10.1007/s00228-020-02879-z. [8] OZEKI T, NAGAHAMA M, FUJITA K, et al. Influence of CYP3A4/5 and ABC transporter polymorphisms on lenvatinib plasma trough concentrations in Japanese patients with thyroid cancer[J]. Sci Rep, 2019, 9( 1): 5404. DOI: 10.1038/s41598-019-41820-y. [9] YANG XR, SUN HC, XIE Q, et al. Chinese expert guidance on overall application of lenvatinib in hepatocellular carcinoma[J]. Chin J Dig Surg, 2023, 22( 2): 167- 180. DOI: 10.3760/cma.j.cn115610-20230201-00035.杨欣荣, 孙惠川, 谢青, 等. 仑伐替尼肝癌全病程应用中国专家指导意见[J]. 中华消化外科杂志, 2023, 22( 2): 167- 180. DOI: 10.3760/cma.j.cn115610-20230201-00035. [10] FOGLI S, GIANFILIPPO G, CUCCHIARA F, et al. Clinical pharmacology and drug-drug interactions of lenvatinib in thyroid cancer[J]. Crit Rev Oncol Hematol, 2021, 163: 103366. DOI: 10.1016/j.critrevonc.2021.103366. [11] KIM BH, YU SJ, KANG W, et al. Expert consensus on the management of adverse events in patients receiving lenvatinib for hepatocellular carcinoma[J]. J Gastroenterol Hepatol, 2022, 37( 3): 428- 439. DOI: 10.1111/jgh.15727. [12] WALIANY S, SAINANI KL, PARK LS, et al. Increase in blood pressure associated with tyrosine kinase inhibitors targeting vascular endothelial growth factor[J]. JACC CardioOncol, 2019, 1( 1): 24- 36. DOI: 10.1016/j.jaccao.2019.08.012. [13] CHEN SJ, KUANG ZM, YUAN H, et al. Advances in research on interactions between amlodipine and other drugs[J]. Chin J Clin Pharmacol Ther, 2014, 19( 6): 701- 706.陈沈珏, 匡泽民, 袁洪, 等. 氨氯地平与其他药物的相互作用研究进展[J]. 中国临床药理学与治疗学, 2014, 19( 6): 701- 706. [14] ZHU YL, WANG F, LI Q, et al. Amlodipine metabolism in human liver microsomes and roles of CYP3A4/5 in the dihydropyridine dehydrogenation[J]. Drug Metab Dispos, 2014, 42( 2): 245- 249. DOI: 10.1124/dmd.113.055400. [15] NAIK KN, JHAJHARIA K, CHAUDHARY R, et al. Multidrug resistance 1 gene polymorphism in amlodipine-induced gingival enlargement[J]. J Indian Soc Periodontol, 2015, 19( 2): 239- 241. DOI: 10.4103/0972-124X.145837. [16] DARVARI R, BOROUJERDI M. Concentration dependency of modulatory effect of amlodipine on P-glycoprotein efflux activity of doxorubicin: A comparison with tamoxifen[J]. J Pharm Pharmacol, 2004, 56( 8): 985- 991. DOI: 10.1211/0022357043941. [17] TAKARA K, MATSUBARA M, YAMAMOTO K, et al. Differential effects of calcium antagonists on ABCG2/BCRP-mediated drug resistance and transport in SN-38-resistant HeLa cells[J]. Mol Med Rep, 2012, 5( 3): 603- 609. DOI: 10.3892/mmr.2011.734. [18] ZHOU YN, ZHANG BK, LI J, et al. Effect of amlodipine on the pharmacokinetics of tacrolimus in rats[J]. Xenobiotica, 2013, 43( 8): 699- 704. DOI: 10.3109/00498254.2012.756992. [19] KUZUYA T, KOBAYASHI T, MORIYAMA N, et al. Amlodipine, but not MDR1 polymorphisms, alters the pharmacokinetics of cyclosporine A in Japanese kidney transplant recipients[J]. Transplantation, 2003, 76( 5): 865- 868. DOI: 10.1097/01.TP.0000084873.20157.67. [20] CUI YJ, LI Y, FAN LJ, et al. UPLC-MS/MS method for the determination of Lenvatinib in rat plasma and its application to drug-drug interaction studies[J]. J Pharm Biomed Anal, 2021, 206: 114360. DOI: 10.1016/j.jpba.2021.114360. [21] CUI YJ, LI Y, GUO CH, et al. Pharmacokinetic interactions between canagliflozin and sorafenib or lenvatinib in rats[J]. Molecules, 2022, 27( 17): 5419. DOI: 10.3390/molecules27175419. [22] CUI YJ, MA YL, LI Y, et al. Influence of schisantherin A on the pharmacokinetics of lenvatinib in rats and its potential mechanism[J]. J Gastrointest Oncol, 2022, 13( 2): 802- 811. DOI: 10.21037/jgo-22-174. [23] CUI YJ, LI Y, LI X, et al. A simple UPLC/MS-MS method for simultaneous determination of lenvatinib and telmisartan in rat plasma, and its application to pharmacokinetic drug-drug interaction study[J]. Molecules, 2022, 27( 4): 1291. DOI: 10.3390/molecules27041291. [24] Center for Drug Evaluation, NMPA. Technical guidelines for drug interaction studies(Trial)[EB/OL].( 2021-01-26)[ 2024-03-12]. https://www.cde.org.cn/main/news/viewInfoCommon/5a15b727e605482c1cf594c689bb994b. https://www.cde.org.cn/main/news/viewInfoCommon/5a15b727e605482c1cf594c689bb994b国家药品监督管理局药品审评中心. 药物相互作用研究技术指导原则(试行)[EB/OL].( 2021-01-26)[ 2024-03-12]. https://www.cde.org.cn/main/news/viewInfoCommon/5a15b727e605482c1cf594c689bb994b. https://www.cde.org.cn/main/news/viewInfoCommon/5a15b727e605482c1cf594c689bb994b -



 PDF下载 ( 1381 KB)
PDF下载 ( 1381 KB)

 下载:
下载: