晚期肝细胞癌局部治疗联合系统治疗后持续5年完全缓解患者的临床特征及影响因素分析
DOI: 10.12449/JCH250818
Clinical features and influencing factors of patients with advanced hepatocellular carcinoma achieving five-year sustained complete remission after local treatment combined with systemic therapy
-
摘要:
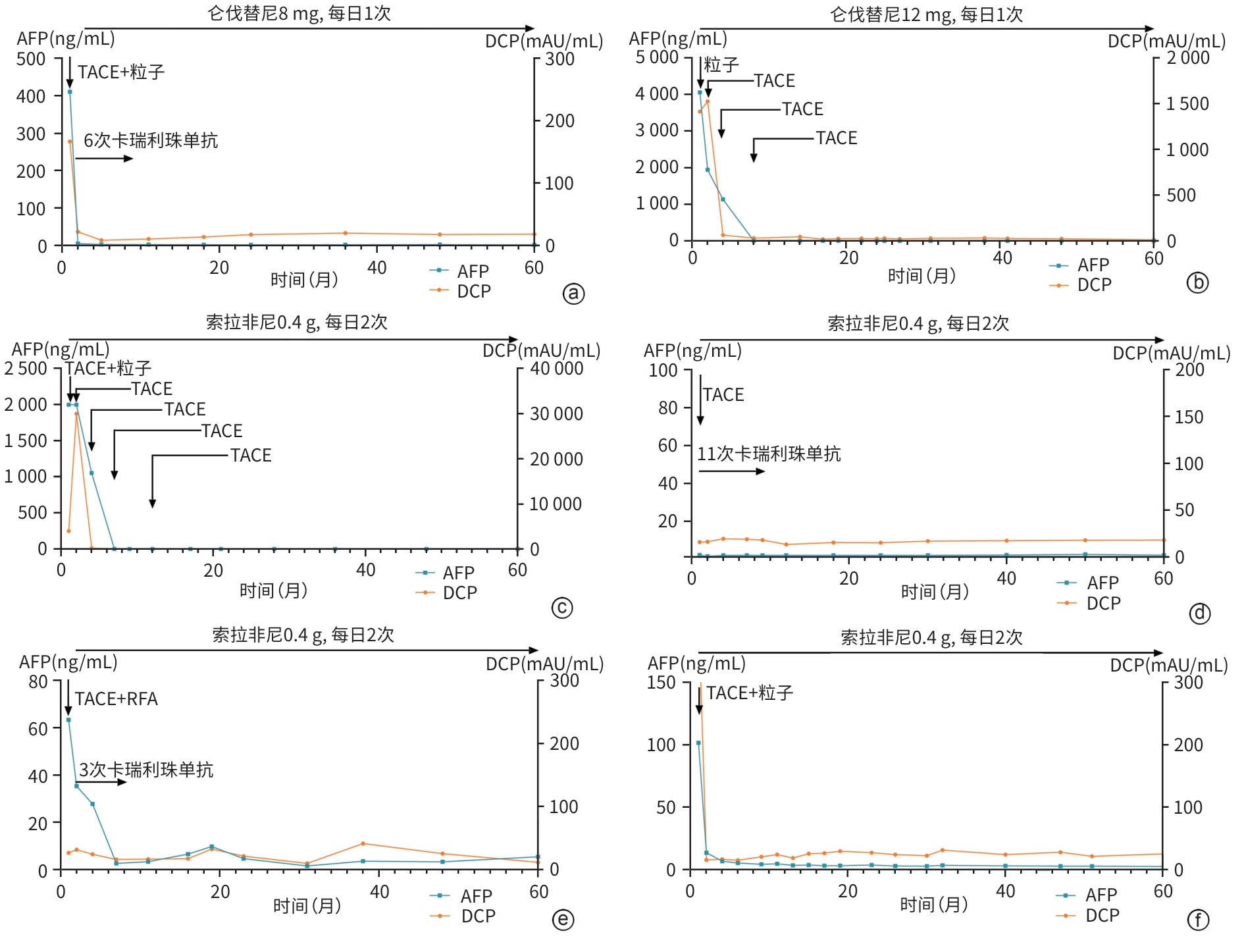
目的 分析中国肝癌分期(CNLC)Ⅲ期肝细胞癌(HCC)患者接受局部治疗联合系统治疗后持续5年完全缓解(CR)的临床特征及影响因素,为晚期HCC治疗策略优化提供参考。 方法 回顾性分析2016年1月—2019年12月在苏州大学附属第一医院介入科接受治疗并持续5年CR的6例CNLCⅢ期HCC患者的临床资料,分析其基线特征、治疗方式及随访数据并进行文献复习。 结果 6例患者平均年龄(58.3±10.1)岁,5例为Ⅲa期,1例为Ⅲb期,均合并肝炎病史,6例患者术前终末期肝病模型评分为(8.2±0.8)分,5例患者术前肝功能Child-Pugh A级,1例为Child-Pugh B级。所有患者接受经导管动脉化疗栓塞术,术后序贯靶向药物治疗,其中索拉非尼4例,仑伐替尼2例。4例合并门静脉主干癌栓的患者联合125I粒子植入,1例单结节型联合射频消融术,3例联合免疫治疗,均为卡瑞利珠单抗。AFP水平复常的中位时间为6个月,治疗至CR的中位时间为5.5个月,中位随访时间为63个月。 结论 良好的肝功能基础、AFP水平早期快速下降、局部治疗与系统治疗的联合模式是晚期HCC患者获得长期CR的关键因素,未来需通过多中心、大样本研究进一步探索其预后影响因素和优化治疗方案。 Abstract:Objective To investigate the clinical features of patients with China Liver Cancer Staging (CNLC) stage Ⅲ hepatocellular carcinoma (HCC) achieving five-year sustained complete remission (CR) after local treatment combined with systemic therapy, as well as potential contributing factors, and to provide a reference for optimizing the treatment of advanced HCC. Methods A retrospective analysis was performed for the clinical data of six patients with CNLC stage Ⅲ HCC who were treated in Department of Interventional Radiology, The First Affiliated Hospital of Soochow University, from January 2016 to December 2019 and achieved five-year sustained CR. Baseline characteristics, treatment modalities, and follow-up data were summarized, and a literature review was performed. Results The six patients had a mean age of 58.3±10.1 years, among whom five had stage Ⅲa HCC and one had stage Ⅲb HCC, and all patients had a history of hepatitis. The mean preoperative MELD score was 8.2±0.8 for the six patients, and there were five patients with Child-Pugh class A liver function and one with Child-Pugh class B liver function. All patients underwent transcatheter arterial chemoembolization, followed by sequential targeted drug therapy after surgery, with sorafenib for four patients and lenvatinib for two patients. Four patients with main portal vein tumor thrombus also received 125I seed implantation, one patient with the single-nodule type underwent radiofrequency ablation, and three patients received immunotherapy with camrelizumab. The median time to AFP normalization was 6 months, the median time from treatment to CR was 5.5 months, and the median follow-up time was 63 months. Conclusion Good liver function at baseline, an early and rapid reduction in AFP, and the combination of local treatment and systemic therapy are key factors for achieving long-term CR in patients with advanced HCC. Multi-center large-scale studies are needed in the future to further explore prognostic factors and optimize treatment regimens. -
Key words:
- Carcinoma, Hepatocellular /
- Local Therapy /
- Systemic Therapy /
- Prognosis
-
表 1 6例CR患者的一般资料及治疗方式
Table 1. Baseline characteristics and treatment of 6 CR patients
患者 性别 年龄
(岁)病因 Child-
Pugh分
级(级)MELD
评分
(分)CNLC
分期血管侵犯 远处
转移大体病理
类型肿瘤
最大径
(cm)肿瘤
数量局部
治疗靶向药物 免疫
治疗至CR
时间
(月)随访
时间
(月)1 女 64 HCV B 8 Ⅲa 门静脉主干
及左右支
下腔静脉无 多结节型 4.5 多发 TACE+
粒子仑伐替尼 卡瑞利
珠单抗2 62 2 男 42 HBV A 9 Ⅲa 门静脉主干
及左右支无 弥漫型 6.7 多发 TACE+
粒子仑伐替尼 无 8 61 3 男 56 HBV A 9 Ⅲa 门静脉主干
及右支无 弥漫型 14.8 多发 TACE+
粒子索拉非尼 无 9 60 4 男 72 HBV A 7 Ⅲb 无 淋巴结 多结节型 7.0 多发 TACE 索拉非尼 卡瑞利
珠单抗4 66 5 男 55 HBV A 8 Ⅲa 门静脉左支 无 单结节型 1.5 单发 TACE+
RFA索拉非尼 卡瑞利
珠单抗7 64 6 男 61 HBV A 8 Ⅲa 门静脉主干
及左右支无 弥漫型 11.0 多发 TACE+
粒子索拉非尼 无 4 96 注:TACE,经导管动脉化疗栓塞术;RFA,射频消融术。
表 2 6例CR患者治疗前后变化
Table 2. Changes before and after treatment in 6 CR patients
患者 AFP(ng/mL) AFP水平复常时间 DCP(mAU/mL) DCP水平复常时间 门静脉通畅情况 肝动脉-门静脉瘘 治疗前 治疗后 治疗前 治疗后 治疗前 治疗后 治疗前 治疗后 1 410.9 5.7 2个月 166.9 22.0 2个月 严重狭窄 通畅 有 无 2 4 059.5 3.3 8个月 1 413.7 31.1 8个月 阻塞 通畅 无 无 3 2 000.0 2.9 6个月 4 015.7 52.0 9个月 阻塞 通畅 有 无 4 2.2 1.5 阴性 16.2 16.6 阴性 通畅 通畅 无 无 5 63.5 2.7 7个月 27.0 16.3 阴性 通畅 通畅 无 无 6 102.2 7.2 2个月 418.6 10.1 3个月 严重狭窄 通畅 无 无 表 3 晚期HCC经治疗后CR病例报道的文献调研结果
Table 3. Results of literature review on CR cases after treatment for advanced HCC
作者 年份 性
别年龄
(岁)病因 Child-
Pugh分
级(级)血管侵犯 肝外
转移局部治疗 抗血管
生成治疗免疫抑制
剂治疗疗效评价
方式AFP正
常化时
间(月)至CR
时间
(月)Sano S[6] 2019 男 75 ALD A 门静脉+
下腔静脉无 HAIC 索拉非尼 无 病理 21 21 Liu Z[7] 2019 男 63 HBV B 门静脉 淋巴结 TACE 仑伐替尼 帕博利珠
单抗mRECIST 4 9 Hirose S[8] 2020 女 69 HCV A 无 肺 无 索拉非尼 无 RECIST 5 5 Ando Y[9] 2020 男 64 HCV B 无 淋巴结 无 索拉非尼 帕博利珠
单抗mRECIST 1.5 3 Goh MJ[10] 2020 男 56 HBV A 门静脉 肺 TARE,TACE 索拉非尼 无 RECIST 28 12 Hwang SY[11] 2021 男 74 ALD A 无 淋巴结 TACE 贝伐珠单抗 阿替利珠
单抗RECIST 4 4 Endo K[12] 2021 女 78 NAFLD A 门静脉 无 TACE 仑伐替尼 无 病理 8 23 Tsai KF[13] 2021 男 63 HBV A 下腔静脉+
右心房肺 HAIC,
dTACE索拉非尼 纳武利尤
单抗mRECIST 21 21 Nong X[14] 2022 男 47 HBV A 门静脉 无 TACE 仑伐替尼 帕博利珠
单抗mRECIST 3 9 Zhong K[15] 2022 男 59 HBV A 门静脉 无 TACE 索拉非尼
瑞戈非尼卡瑞利珠
单抗mRECIST 11 13 Zhao Y[16] 2023 男 51 HBV A 门静脉+
下腔静脉无 放疗 仑伐替尼 帕博利珠
单抗RECIST 6 6 Tomonari T[17] 2023 男 50 HBV A 门静脉 无 TACE 仑伐替尼 无 RECIST 12 12 Kurisaki K[18] 2023 男 60 HBV A 门静脉 无 无 贝伐珠单抗 阿替利珠
单抗病理 2 4 Belén MB[19] 2024 女 67 HCV,
AILDA 门静脉 无 TARE 仑伐替尼 无 mRECIST 3 6 Okuno K[20] 2024 男 83 ALD A 门静脉 淋巴结 无 贝伐珠单抗 阿替利珠
单抗病理 2 5 Qiao JH[21] 2024 女 50 HBV A 门静脉 淋巴结 HAIC 阿帕替尼 卡瑞利珠
单抗mRECIST 5 9 Xiao XH[22] 2024 女 57 HBV A 门静脉 无 TACE 多纳非尼 替雷利珠
单抗mRECIST 7 7 Arima S[23] 2024 男 80 ALD A 无 淋巴结 无 贝伐珠单抗 阿替利珠
单抗mRECIST 5 5 注:ALD,酒精性肝病;NAFLD,非酒精性脂肪性肝病;AILD,自身免疫性肝病;HAIC,肝动脉灌注化疗;TARE,经动脉放射栓塞;dTACE,载药微球栓塞。
-
[1] HAN BF, ZHENG RS, ZENG HM, et al. Cancer incidence and mortality in China, 2022[J]. J Natl Cancer Cent, 2024, 4( 1): 47- 53. DOI: 10.1016/j.jncc.2024.01.006. [2] Alliance of Liver Cancer Conversion Therapy, Committee of Liver Cancer, China Anti-Cancer Association. Chinese expert consensus on conversion and perioperative therapy of primary liver cancer(2024 edition)[J]. Chin J Dig Surg, 2024( 4): 492- 513. DOI: 10.3760/cma.j.cn115610-20240228-00135.中国抗癌协会肝癌专业委员会转化治疗协作组. 原发性肝癌转化及围手术期治疗中国专家共识(2024版)[J]. 中华消化外科杂志, 2024( 4): 492- 513. DOI: 10.3760/cma.j.cn115610-20240228-00135. [3] HUANG ZJ, ZHANG LD, WU ZY, et al. Conversion therapy for unresectable hepatocellular carcinoma[J/OL]. Chin J Hepat Surg(Electronic Edition), 2025, 14( 1): 41- 45.黄忠晶, 张丽东, 伍子奕, 等. 不可切除肝细胞癌的转化治疗[J/OL]. 中华肝脏外科手术学电子杂志, 2025, 14( 1): 41- 45. [4] National Health Commission of the People’s Republic of China. Standard for diagnosis and treatment of primary liver cancer(2024 edition)[J]. J Clin Hepatol, 2024, 40( 5): 893- 918. DOI: 10.12449/JCH240508.中华人民共和国国家卫生健康委员会. 原发性肝癌诊疗指南(2024年版)[J]. 临床肝胆病杂志, 2024, 40( 5): 893- 918. DOI: 10.12449/JCH240508. [5] LLOVET JM, LENCIONI R. mRECIST for HCC: Performance and novel refinements[J]. J Hepatol, 2020, 72( 2): 288- 306. DOI: 10.1016/j.jhep.2019.09.026. [6] SANO S, NAKATA S, WADA SC, et al. Pathological complete response by advanced hepatocellular carcinoma with massive macrovascular invasion to hepatic arterial infusion chemotherapy: A case report[J]. World J Surg Oncol, 2019, 17( 1): 229. DOI: 10.1186/s12957-019-1772-8. [7] LIU ZN, LI XJ, HE XQ, et al. Complete response to the combination of Lenvatinib and Pembrolizumab in an advanced hepatocellular carcinoma patient: A case report[J]. BMC Cancer, 2019, 19( 1): 1062. DOI: 10.1186/s12885-019-6287-8. [8] HIROSE S, ISHIGE K, YAMAURA M, et al. A case report: Long-term complete response of metastatic hepatocellular carcinoma obtained after discontinuation of 2-month sorafenib monotherapy[J]. Clin J Gastroenterol, 2020, 13( 5): 902- 906. DOI: 10.1007/s12328-020-01154-z. [9] ANDO Y, YAMAUCHI M, SUEHIRO Y, et al. Complete response to pembrolizumab in advanced hepatocellular carcinoma with microsatellite instability[J]. Clin J Gastroenterol, 2020, 13( 5): 867- 872. DOI: 10.1007/s12328-020-01099-3. [10] GOH MJ, KANG W, SINN DH, et al. Advanced stage hepatocellular carcinoma successfully treated with transarterial radioembolization and multi-tyrosine kinase inhibitor therapy[J]. J Liver Cancer, 2020, 20( 2): 160- 166. DOI: 10.17998/jlc.20.2.160. [11] HWANG SY, LEE SM, LIM JW, et al. Complete response in hepatocellular carcinoma with lymph node metastasis by combination therapy of atezolizumab and bevacizumab: A case report[J]. J Liver Cancer, 2021, 21( 2): 177- 180. DOI: 10.17998/jlc.2021.09.10. [12] ENDO K, KURODA H, ABE T, et al. Two hepatectomy cases for initially unresectable hepatocellular carcinoma after achieving a radiological complete response to sequential therapy with lenvatinib and transcatheter arterial chemoembolization[J]. Hepatol Res, 2021, 51( 10): 1082- 1086. DOI: 10.1111/hepr.13665. [13] TSAI KF, TSAI JCH, LI MF, et al. Complete response in metastatic hepatocellular carcinoma with cardiac and lung involvement via multimodality treatment[J]. Medicina(Kaunas), 2021, 57( 8): 849. DOI: 10.3390/medicina57080849. [14] NONG X, ZHANG YM, LIANG JC, et al. Complete response by patients with advanced hepatocellular carcinoma after combination immune/targeted therapy and transarterial chemoembolization: Two case reports and literature review[J]. Transl Cancer Res, 2022, 11( 8): 2973- 2984. DOI: 10.21037/tcr-21-2691. [15] ZHONG KH, XU YY, CHENG Y, et al. Case report: Primary hepatocellular carcinoma with portal vein tumor Thrombus characterized by active tumor immune microenvironment achieving a complete response following treatment of combined immunotherapy[J]. Front Immunol, 2022, 13: 999763. DOI: 10.3389/fimmu.2022.999763. [16] ZHAO Y, HE GS, LI G. Triplet regimen as a novel modality for advanced unresectable hepatocellular carcinoma: A case report and review of literature[J]. World J Clin Cases, 2023, 11( 27): 6558- 6564. DOI: 10.12998/wjcc.v11.i27.6558. [17] TOMONARI T, TANAKA H, TANAKA T, et al. A case of complete response with rechallenge-lenvatinib plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma refractory to multiple molecular-targeted agent treatments[J]. Clin J Gastroenterol, 2023, 16( 3): 438- 443. DOI: 10.1007/s12328-023-01777-y. [18] KURISAKI K, SOYAMA A, HARA T, et al. Pathologic complete response after chemotherapy with atezolizumab plus bevacizumab for hepatocellular carcinoma with tumor Thrombus in the main portal trunk[J]. Dig Surg, 2023, 40( 1-2): 84- 89. DOI: 10.1159/000529405. [19] MARTÍNEZ BENITO B, GONZÁLEZ PINTOR V, DEL CAMPO DEL VAL L, et al. Complete tumor response after concomitant treatment with lenvatinib and radioembolization with Ytrio-90 of advanced stage hepatocarcinoma[J]. Gastroenterol Hepatol, 2024, 47( 6): 620- 622. DOI: 10.1016/j.gastrohep.2023.09.005. [20] OKUNO M, OHAMA H, NAKAMURA I, et al. A patient who underwent conversion surgery after atezolizumab plus bevacizumab for hepatocellular carcinoma with portal vein thrombosis and perihepatic lymph node metastases achieved a pathological complete response[J]. Int Cancer Conf J, 2024, 13( 3): 306- 312. DOI: 10.1007/s13691-024-00683-5. [21] QIAO JH, WANG Y, FU CX, et al. Complete response and long-term survival after short-course camrelizumab plus apatinib, hepatic arterial infusion chemotherapy, and transarterial chemoembolization in large and advanced hepatocellular carcinoma: A case report[J]. J Gastrointest Oncol, 2024, 15( 5): 2323- 2329. DOI: 10.21037/jgo-24-613. [22] XIAO XH, FU HX, QIN HX, et al. Case report: Complete response after transcatheter arterial chemoembolization combined with donafenib plus tislelizumab therapy for hepatocellular carcinoma with main trunk portal vein tumor Thrombus in a patient coinfected with HIV and HBV[J]. Front Immunol, 2024, 15: 1422801. DOI: 10.3389/fimmu.2024.1422801. [23] ARIMA S, KANDA T, TOTSUKA M, et al. Elderly patient with unresectable advanced-stage hepatocellular carcinoma who received atezolizumab plus bevacizumab and achieved a complete response: A case report[J]. Med Int(Lond), 2024, 4( 3): 23. DOI: 10.3892/mi.2024.147. [24] FAN J, GAO Q. Immunotherapy for hepacellular carcinoma: Where there is hope, there is brightness[J]. Chin J Dig Surg, 2022, 21( 2): 199- 204. DOI: 10.3760/cma.j.cn115610-20220215-00080.樊嘉, 高强. 肝癌的免疫治疗: 有希望便是光明[J]. 中华消化外科杂志, 2022, 21( 2): 199- 204. DOI: 10.3760/cma.j.cn115610-20220215-00080. [25] PINTER M, SIEGHART W. Long-term remission in advanced stage hepatocellular carcinoma? Achance for cure?[J]. Memo Mag Eur Med Oncol, 2018, 11( 3): 185- 192. DOI: 10.1007/s12254-018-0431-z. [26] RIMOLA J, DÍAZ-GONZÁLEZ Á, DARNELL A, et al. Complete response under sorafenib in patients with hepatocellular carcinoma: Relationship with dermatologic adverse events[J]. Hepatology, 2018, 67( 2): 612- 622. DOI: 10.1002/hep.29515. [27] CABIBBO G, MAIDA M, GENCO C, et al. Survival of patients with hepatocellular carcinoma(HCC) treated by percutaneous radio-frequency ablation(RFA) is affected by complete radiological response[J]. PLoS One, 2013, 8( 7): e70016. DOI: 10.1371/journal.pone.0070016. [28] YOUNES EH, ZAHRA HF, SOUMAYA BM, et al. Study of predictive factors of complete response after chemoembolization for unresectable hepatocellular carcinoma in 162 patients[J]. Clin Exp Hepatol, 2020, 6( 4): 313- 320. DOI: 10.5114/ceh.2020.102169. [29] Liver Oncology Branch of China Association for the Promotion of International Healthcare Exchanges, Multi-disciplinary Collaboration Group of Digestive Tract Oncology, Cancer Hospital of Peking Union Medical College, Chinese Academy of Medical Sciences, and Liver Cancer Professional Committee of Chinese Medical Doctor Association. Expert consensus on whole course management of liver function in liver cancer transformation therapy(2022 edition)[J]. Electron J Liver Tumor, 2023, 10( 1): 1- 9.中国医疗保健国际交流促进会肝脏肿瘤学分会, 中国医学科学院北京协和医院肿瘤医院消化道肿瘤多学科协作组, 中国医师协会肝癌专业委员会. 肝癌转化治疗中肝功能全程管理中国专家共识(2022版)[J]. 肝癌电子杂志, 2023, 10( 1): 1- 9. [30] KUZUYA T, KAWABE N, MUTO H, et al. Characteristics and prognosis of patients with advanced hepatocellular carcinoma treated with atezolizumab/bevacizumab combination therapy who achieved complete response[J]. Curr Oncol, 2024, 31( 10): 6218- 6231. DOI: 10.3390/curroncol31100463. [31] KUZUYA T, KAWABE N, HASHIMOTO S, et al. Early changes in alpha-fetoprotein are a useful predictor of efficacy of atezolizumab plus bevacizumab treatment in patients with advanced hepatocellular carcinoma[J]. Oncology, 2022, 100( 1): 12- 21. DOI: 10.1159/000519448. [32] XU JH, YIN DX, LI YC, et al. Systemic therapy for advanced hepatocellular carcinoma[J]. J Clin Hepatol, 2024, 40( 11): 2306- 2314. DOI: 10.12449/JCH241127.徐家豪, 尹东旭, 李宇辰, 等. 晚期肝细胞癌的系统治疗[J]. 临床肝胆病杂志, 2024, 40( 11): 2306- 2314. DOI: 10.12449/JCH241127. [33] KUANG M. Advances in neoadjuvant therapy for hepatocellular carcinoma[J]. Chin J Dig Surg, 2023, 22( 2): 202- 208. DOI: 10.3760/cma.j.cn115610-20221203-00728.匡铭. 肝细胞癌新辅助治疗研究进展[J]. 中华消化外科杂志, 2023, 22( 2): 202- 208. DOI: 10.3760/cma.j.cn115610-20221203-00728. [34] DING D, FU SZ, DAI F, et al. Therapeutic effect of intervention combined with sorafenib in the treatment of hepatocellular carcinoma with main portal vein tumor Thrombus[J]. Chin Comput Med Imag, 2023, 29( 4): 433- 437. DOI: 10.19627/j.cnki.cn31-1700/th.20230329.003.丁丁, 付守忠, 戴锋, 等. 介入联合索拉菲尼治疗肝癌伴门脉癌栓的疗效观察[J]. 中国医学计算机成像杂志, 2023, 29( 4): 433- 437. DOI: 10.19627/j.cnki.cn31-1700/th.20230329.003. [35] Liver Cancer Committee, Chinese Medical Doctor Association. Guidelines for diagnosis and treatment of hepatocellular carcinoma complicated with portal vein cancer thrombus in China(2021 edition)[J]. Natl Med J China, 2022, 102( 4): 243- 254. DOI: 10.3760/cma.j.cn112137-20211117-02567.中国医师协会肝癌专业委员会. 中国肝细胞癌合并门静脉癌栓诊疗指南(2021年版)[J]. 中华医学杂志, 2022, 102( 4): 243- 254. DOI: 10.3760/cma.j.cn112137-20211117-02567. [36] JIN ZC, CHEN JJ, ZHU XL, et al. Immune checkpoint inhibitors and anti-vascular endothelial growth factor antibody/tyrosine kinase inhibitors with or without transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma(CHANCE2201): A target trial emulation study[J]. EClinicalMedicine, 2024, 72: 102622. DOI: 10.1016/j.eclinm.2024.102622. [37] SANGRO B, KUDO M, ERINJERI JP, et al. Durvalumab with or without bevacizumab with transarterial chemoembolisation in hepatocellular carcinoma(EMERALD-1): A multiregional, randomised, double-blind, placebo-controlled, phase 3 study[J]. Lancet, 2025, 405( 10474): 216- 232. DOI: 10.1016/S0140-6736(24)02551-0. -



 PDF下载 ( 2144 KB)
PDF下载 ( 2144 KB)

 下载:
下载:



