慢加急性肝衰竭患者预后预测评分表的构建及验证
DOI: 10.12449/JCH251021
Construction and validation of a novel prognostic risk scoring table for patients with acute-on-chronic liver failure
-
摘要:
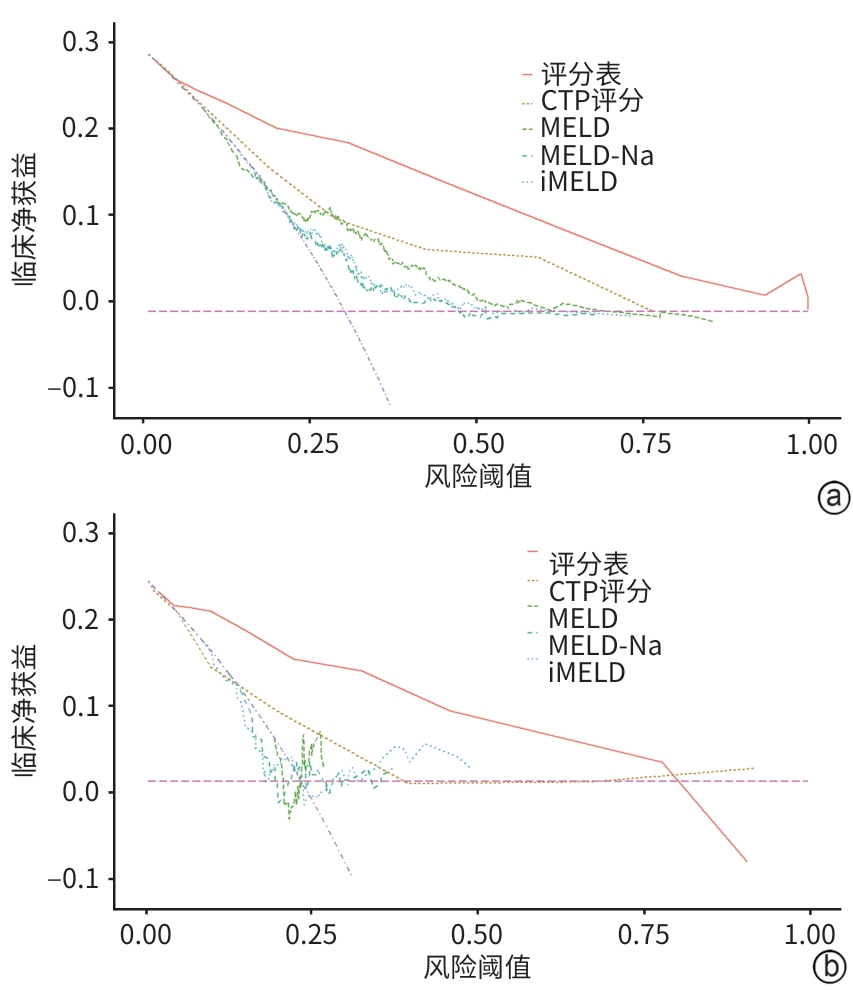
目的 探讨慢加急性肝衰竭(ACLF)患者的临床特征,构建一种能够在早期准确预测患者预后的风险评分表。 方法 回顾性分析2010年1月1日—2020年12月31日空军军医大学唐都医院收治的502例ACLF患者的临床资料(训练集),筛选与入院28天病死率相关的影响因素。将2021年1月1日—2021年12月31日空军军医大学唐都医院收治的69例ACLF患者作为验证集。计量资料两组间比较采用成组t检验或Mann-Whitney U检验。计数资料两组间比较采用χ2检验或Fisher精确概率法。应用单因素Cox回归初步筛选与ACLF患者入院28天预后相关的预测指标,使用方差膨胀因子对预测指标进行多重共线性分析,通过多因素Cox回归分析构建ACLF预后(死亡)风险模型。基于构建方程中各项指标的回归系数β及列线图中各项指标的权重,建立ACLF预后(死亡)风险评分表。分别在训练集中对ACLF预后(死亡)风险模型、ACLF预后(死亡)风险评分表及其他评分模型[包括Child-Turcotte-Pugh(CTP)评分、终末期肝病模型(MELD)、终末期肝病模型钠(MELD-Na)、终末期肝病综合模型(iMELD)]进行内部验证和比较,在验证集中对ACLF预后(死亡)风险评分表和其他评分模型进行外部验证并综合评价。使用Nagelkerke R2和Hosmer-Lemeshow检验评价ACLF预后(死亡)风险模型、ACLF预后(死亡)风险评分表及其他评分模型的拟合度,并绘制拟合曲线。使用C指数评价ACLF预后(死亡)风险评分表与其他评分模型的区分度,并应用Z检验比较不同模型间C指数的差异。使用决策曲线分析(DCA)比较ACLF预后(死亡)风险评分表和其他评分模型的临床获益。 结果 多因素Cox回归分析确定年龄(HR=1.027,95%CI:1.015~1.039,P<0.001)、肝性脑病分级(1级:HR=2.928,95%CI:1.463~5.858,P=0.002;2级:HR=3.811,95%CI:2.078~6.988,P<0.001;3级:HR=3.916,95%CI:1.917~8.001,P<0.001;4级:HR=6.966,95%CI:4.559~10.644,P<0.001)、TBil日上升≥17.1 μmol/L(HR=1.771,95%CI:1.248~2.513,P=0.001)、肌酐(HR=1.005,95%CI:1.004~1.006,P<0.001)、中性粒细胞计数(HR=1.092,95%CI:1.060~1.126,P<0.001)、国际标准化比值(HR=1.298,95%CI:1.187~1.418,P<0.001)是与ACLF患者入院28天病死率显著相关的独立危险因素,构建ACLF预后(死亡)风险评分表。Nagelkerke R2检验结果显示,在训练集和验证集中,ACLF预后(死亡)风险评分表的R2值分别为0.599和0.722,高于CTP评分、MELD、MELD-Na和iMELD。Hosmer-Lemeshow检验结果显示,在训练集和验证集中,ACLF预后(死亡)风险评分表的P值分别为0.280和0.788。C指数分析结果显示,在训练集中,评分表的C指数高于CTP评分,差异有统计学意义(P<0.001);在验证集中,评分表的C指数高于其他模型评分,差异均有统计学意义(P值均<0.001)。DCA结果显示,使用ACLF预后(死亡)风险评分表较其他评分模型的临床净获益高。 结论 与目前临床使用的其他评分模型相比,基于年龄、肝性脑病分级、TBil日上升≥17.1 μmol/L、肌酐、中性粒细胞计数和国际标准化比值6个预测因素所构建的ACLF预后(死亡)风险评分表对ACLF患者入院28天预后具有较高的预测价值。 Abstract:Objective To investigate the clinical features of patients with acute-on-chronic liver failure (ACLF), and to construct a risk scoring table that can accurately predict the prognosis of patients in the early stage. Methods A retrospective analysis was performed for the clinical data of 502 patients with ACLF who were admitted to Tangdu Hospital, Air Force Medical University, from January 1, 2010 to December 31, 2020 (training set), and the influencing factors for 28-day mortality rate were identified. The 69 ACLF patients who were admitted to Tangdu Hospital, Air Force Medical University, from January 1 to December 31, 2021 were enrolled as the validation set. The independent-samples t test or the Mann-Whitney U test was used for comparison of continuous data between two groups, and the chi-square test or the Fisher’s exact test was used for comparison of categorical data between two groups. A univariate Cox regression analysis was used to obtain the early warning indicators associated with the 28-day prognosis of ACLF patients, and variance inflation factors were used to assess multicollinearity among predictors; a multivariate Cox regression analysis was used to construct a risk model for ACLF prognosis (mortality). A risk scoring table for ACLF prognosis (mortality) was developed based on regression coefficients (β) from the model equation and weight assignments in the nomogram. Internal validation and comparison were performed for the risk model for ACLF prognosis (mortality), the scoring table for ACLF prognosis (mortality), and other scoring models (Child-Turcotte-Pugh [CTP] score, Model for End-Stage Liver Disease [MELD] score, MELD combined with serum sodium concentration [MELD-Na] score, and integrated MELD [iMELD] score) in the training set, while external validation and comprehensive evaluation of the scoring table and the other scoring models were performed in the validation set. The Nagelkerke’s R2 test and the Hosmer-Lemeshow test were used to assess the degree of fitting of the risk model for ACLF prognosis (mortality), the scoring table for ACLF prognosis (mortality), and other scoring models, and fitting curves were plotted. C-index was used to assess the discriminatory ability of the scoring table for ACLF prognosis (mortality) and the other scoring models, and the Z-test was used for comparison of C-index between different models. The decision curve analysis was used to compare the clinical benefits of the scoring table for ACLF prognosis (mortality) and the other scoring models. Results The multivariate Cox regression analysis showed that age (hazard ratio [HR]=1.027, 95% confidence interval [CI]: 1.015 — 1.039, P<0.001), hepatic encephalopathy grade (grade 1: HR=2.928, 95%CI: 1.463 — 5.858, P=0.002; grade 2: HR=3.811, 95%CI: 2.078 — 6.988, P<0.001; grade 3: HR=3.916, 95%CI: 1.917 — 8.001, P<0.001; grade 4: HR=6.966, 95%CI: 4.559 — 10.644, P<0.001), an increase in total bilirubin (TBil) by ≥17.1 μmol/L per day (HR=1.771, 95%CI: 1.248 — 2.513, P=0.001), creatinine (HR=1.005, 95%CI: 1.004 — 1.006, P<0.001), neutrophil count (HR=1.092, 95%CI: 1.060 — 1.126, P<0.001), and international normalized ratio (HR=1.298, 95%CI: 1.187 — 1.418, P<0.001) were independent risk factors associated with the 28-day mortality rate of ACLF patients, and a risk scoring table was constructed for ACLF prognosis (mortality). The Nagelkerke’s R2 test showed that the risk scoring table for ACLF prognosis (mortality) had an R2 value of 0.599 in the training set and 0.722 in the validation set, which were higher than the R2 values of CTP, MELD, MELD-Na, and iMELD scores. The Hosmer-Lemeshow test showed that the risk scoring table for ACLF prognosis (mortality) had a P value of 0.280 in the training set and 0.788 in the validation set. The C-index analysis showed that the scoring table had a higher C-index than the other scoring models in the validation set (all P<0.001), as well as a higher C-index than CTP score in the training set (P<0.001). The decision curve analysis showed that the risk scoring table for ACLF prognosis (mortality) had higher clinical net benefits than the other scoring models. Conclusion Compared with other scoring models currently used in clinical practice, the novel risk scoring table for ACLF prognosis (mortality) constructed based on the six predictive factors of age, hepatic encephalopathy grade, an increase in TBil by ≥17.1 μmol/L per day, creatinine, neutrophil count, and international normalized ratio has a relatively high value in predicting the 28-day prognosis of ACLF patients. -
Key words:
- Acute-On-Chronic Liver Failure /
- Proportional Hazards Models /
- Prognosis /
- Risk Factors
-
表 1 ACLF患者的基线资料
Table 1. Patient characteristics at enrolment
基线资料 所有患者(n=502) 死亡组(n=161) 生存组(n=341) 统计值 P值 年龄(岁) 48.18±13.87 50.34±14.19 47.16±13.61 t=2.412 0.016 性别[例(%)] χ2=2.754 0.097 女 169(33.7) 46(27.2) 123(72.8) 男 333(66.3) 115(34.5) 218(65.5) 肝病病因[例(%)] HBV感染 277(55.2) 94(33.9) 183(66.1) χ2=0.985 0.321 胆汁淤积性肝病 87(17.3) 28(32.2) 59(67.8) χ2=0.001 0.980 自身免疫性肝病 47(9.4) 15(31.9) 32(68.1) χ2=0.001 0.981 酒精性肝病 43(8.6) 16(37.2) 27(62.8) χ2=0.570 0.450 药物性肝病 22(4.4) 3(13.6) 19(86.4) χ2=2.759 0.097 HCV感染 13(2.6) 3(23.1) 10(76.9) χ2=0.162 0.687 肝豆状核变性 6(1.2) 1(16.7) 5(83.3) χ2=0.139 0.709 HEV感染 3(0.6) 0(0.0) 3(100.0) 0.555 EBV感染 3(0.6) 1(33.3) 2(66.7) χ2=0.000 >0.05 未明确病因 1(0.2) 0(0.0) 1(100.0) >0.05 慢性肝病基础[例(%)] χ2=0.071 0.791 慢性肝病 81(16.1) 27(33.3) 54(66.7) 肝硬化 421(83.9) 134(31.8) 287(68.2) 并发症[例(%)] 消化道出血 37(7.4) 22(59.5) 15(40.5) χ2=13.753 <0.001 腹水 400(79.7) 135(33.8) 265(66.2) χ2=2.545 0.111 感染 238(47.4) 85(35.7) 153(64.3) χ2=2.756 0.097 肝性脑病分级 χ2=13.787 <0.001 1级 34(6.8) 11(32.4) 23(67.6) 2级 32(6.4) 16(50.0) 16(50.0) 3级 20(4.0) 10(50.0) 10(50.0) 4级 111(22.1) 91(82.0) 20(18.0) 器官衰竭[例(%)] 肾衰竭 86(17.1) 61(70.9) 25(29.1) χ2=71.930 <0.001 呼吸衰竭 44(8.8) 41(93.2) 3(6.8) χ2=79.623 <0.001 循环衰竭 38(7.6) 33(86.8) 5(13.2) χ2=53.922 <0.001 实验室检查 WBC(×109/L) 6.61(4.54~9.77) 8.51(6.29~11.83) 5.87(4.08~8.32) Z=6.467 <0.001 Lym(×109/L) 0.96(0.66~1.42) 0.91(0.63~1.31) 1.00(0.70~1.45) Z=1.585 0.113 Mono(×109/L) 0.64(0.39~0.92) 0.68(0.43~1.11) 0.61(0.38~0.88) Z=1.907 0.056 Neu(×109/L) 4.58(3.07~7.29) 6.88(4.29~9.61) 4.04(2.67~5.87) Z=7.379 <0.001 Hb(g/L) 117.32±26.83 115.25±29.73 118.30±25.34 t=1.123 0.263 PLT(×109/L) 90.00(56.00~128.00) 88.00(51.00~116.00) 92.00(57.00~133.50) Z=1.935 0.053 HCT(%) 33.85±7.45 33.36±8.31 34.07±7.01 t=0.936 0.350 Alb(g/L) 30.20(26.90~33.40) 29.70(26.20~33.15) 30.90(27.20~33.70) Z=2.230 0.026 ALT(U/L) 219.50(63.75~608.50) 288.00(65.50~853.00) 202.00(62.50~570.00) Z=1.778 0.075 AST(U/L) 216.50(97.00~548.50) 260.00(97.50~744.00) 208.00(96.00~484.00) Z=1.382 0.167 TBil(μmol/L) 321.34(221.08~429.85) 334.70(230.95~446.25) 316.94(216.10~415.20) Z=1.800 0.072 血氨(μmol/L) 64.85(42.83~93.58) 81.30(46.48~118.73) 59.25(41.33~83.95) Z=4.300 <0.001 GGT(U/L) 81.50(49.00~139.50) 81.00(44.50~129.00) 84.00(50.00~144.50) Z=1.235 0.217 ALP(U/L) 136.00(108.00~182.00) 133.00(112.50~191.00) 138.00(107.00~179.00) Z=0.525 0.600 Cr(μmol/L) 61.90(51.00~80.00) 71.00(57.70~131.50) 59.00(49.00~72.50) Z=6.551 <0.001 BUN(mmol/L) 4.72(3.25~7.60) 6.60(3.44~13.55) 4.22(3.20~6.40) Z=5.158 <0.001 Na+(mmol/L) 136.40(132.50~139.30) 135.30(130.90~138.90) 136.90(133.35~139.55) Z=2.965 0.003 K+(mmol/L) 4.06(3.53~4.62) 4.23(3.55~4.74) 3.98(3.49~4.54) Z=2.371 0.018 PT(s) 22.25(18.70~28.93) 27.90(20.80~37.80) 20.90(18.10~25.60) Z=7.153 <0.001 APTT(s) 52.00(42.90~61.90) 56.00(44.70~67.50) 50.40(41.65~58.85) Z=3.407 0.001 TT(s) 22.90(20.60~26.00) 23.20(20.60~26.85) 22.80(20.60~25.60) Z=1.397 0.162 Fib(g/L) 1.18(0.87~1.53) 1.09(0.81~1.53) 1.20(0.89~1.53) Z=1.233 0.218 PTA(%) 34.20(24.58~43.73) 25.10(17.65~36.25) 37.00(29.00~46.25) Z=7.457 <0.001 INR 1.97(1.64~2.60) 2.51(1.85~3.39) 1.85(1.60~2.31) Z=7.213 <0.001 FDP(μg/mL) 5.77(3.20~10.63) 7.40(5.00~15.23) 5.00(2.53~9.45) Z=5.229 <0.001 D-D(μg/mL) 2.15(0.68~4.55) 2.84(0.96~6.80) 1.88(0.62~3.87) Z=3.569 <0.001 其他[例(%)] TBil日上升≥17.1 μmol/L 194(38.6) 107(55.2) 87(44.8) χ2=77.333 <0.001 Child-Pugh分级 χ2=22.304 <0.001 A级 22(4.4) 2(9.1) 20(90.9) B级 71(14.1) 10(14.1) 61(85.9) C级 409(81.5) 149(36.4) 260(63.6) 注:EBV,EB病毒;Lym,淋巴细胞计数;Mono,单核细胞计数;Neu,中性粒细胞计数;Hb,血红蛋白;HCT,红细胞比容;Cr,肌酐;BUN,血尿素氮;Na+,血清钠离子;K+,血清钾离子;PT,凝血酶原时间;APTT,活化部分凝血活酶时间;TT,凝血酶时间;Fib,纤维蛋白原;PTA,凝血酶原活动度;INR,国际标准化比值;FDP,纤维蛋白降解产物;D-D,D-二聚体。
表 2 ACLF预后(死亡)风险模型
Table 2. ACLF prognostic (death) risk model
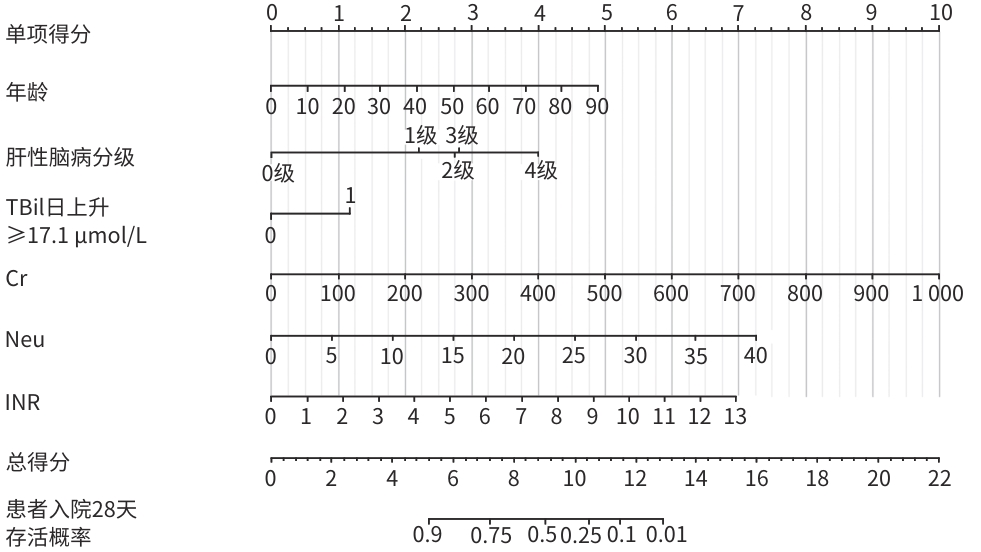
自变量 β值 SE HR(95%CI) Z值 P值 年龄(岁) 0.026 0.006 1.027(1.015~1.039) 4.455 <0.001 肝性脑病分级 1级 1.074 0.354 2.928(1.463~5.858) 3.036 0.002 2级 1.338 0.309 3.811(2.078~6.988) 4.325 <0.001 3级 1.365 0.365 3.916(1.917~8.001) 3.745 <0.001 4级 1.941 0.216 6.966(4.559~10.644) 8.973 <0.001 TBil日上升≥17.1 μmol/L 0.572 0.178 1.771(1.248~2.513) 3.203 0.001 Cr(μmol/L) 0.005 0.001 1.005(1.004~1.006) 7.361 <0.001 Neu(×109/L) 0.088 0.015 1.092(1.060~1.126) 5.808 <0.001 INR 0.261 0.045 1.298(1.187~1.418) 5.744 <0.001 表 3 ACLF预后(死亡)风险评分表
Table 3. ACLF prognostic risk score
预测变量 0分 1分 2分 3分 4分 年龄(岁) ≤10 >10~30 >30~50 >50~70 >70 肝性脑病分级 无 1级 2级或3级 4级 TBil日上升≥17.1 μmol/L 无 有 Cr(μmol/L) ≤100 >100~200 >200~300 >300~400 >400 Neu(×109/L) ≤8 >8~15 >15~20 >20 INR ≤1.2 >1.2~3.0 >3.0~5.0 >5.0~7.0 >7.0 表 4 评分表及其他评分模型的拟合度
Table 4. The fit of the scoring table and other scoring models
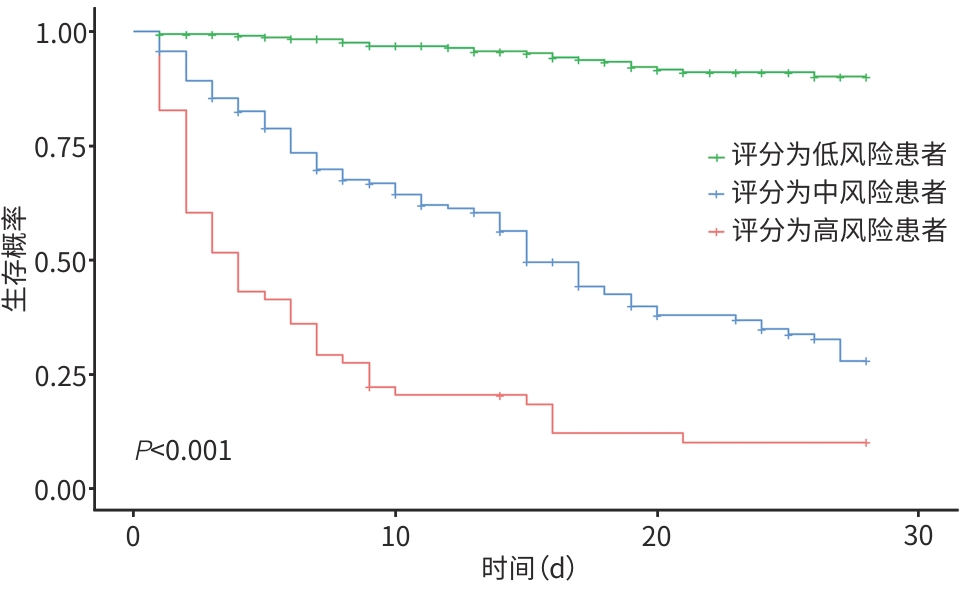
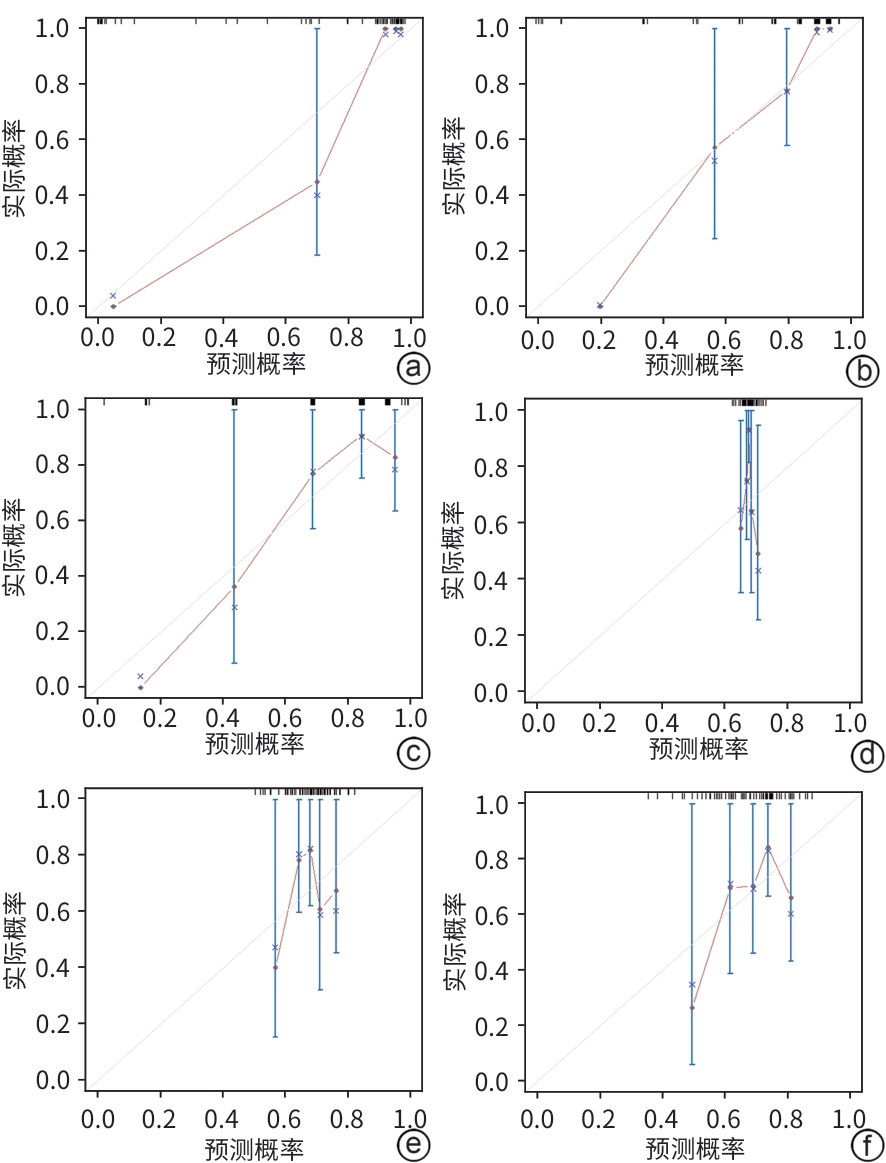
评分模型 训练集 验证集 R2值 P值 R2值 P值 风险模型 0.626 0.917 0.888 0.999 评分表 0.599 0.280 0.722 0.788 CTP评分 0.299 <0.001 0.318 0.035 MELD 0.049 0.844 0.044 0.226 MELD-Na 0.100 0.141 <0.001 0.083 iMELD 0.040 0.958 0.015 0.189 表 5 评分表与其他评分模型对ACLF患者预后的区分度
Table 5. Differentiation of scoring tables and other scoring models on prognosis in patients with ACLF
评分模型 训练集 验证集 C指数(95%CI) Z值 P值1) C指数(95%CI) Z值 P值1) 评分表 0.854(0.840~0.867) 0.900(0.870~0.930) CTP评分 0.684(0.663~0.706) 3.654 <0.001 0.749(0.686~0.812) 7.809 <0.001 MELD 0.733(0.711~0.755) 0.265 0.791 0.552(0.465~0.640) 10.860 <0.001 MELD-Na 0.684(0.662~0.707) 0.987 0.324 0.551(0.466~0.636) 9.076 <0.001 iMELD 0.719(0.697~0.740) 1.603 0.109 0.569(0.480~0.658) 10.810 <0.001 注:1)与评分表比较。
-
[1] BI ZH, WANG LX, LIAN JQ. Definition, prognostic assessment, and advances in the diagnosis and treatment of acute-on-chronic liver failure[J]. J Clin Hepatol, 2022, 38( 7): 1671- 1676. DOI: 10.3969/j.issn.1001-5256.2022.07.041.毕占虎, 王临旭, 连建奇. 慢加急性肝衰竭的定义、预后评估及诊治进展[J]. 临床肝胆病杂志, 2022, 38( 7): 1671- 1676. DOI: 10.3969/j.issn.1001-5256.2022.07.041. [2] BAJAJ JS, O’LEARY JG, LAI JC, et al. Acute-on-chronic liver failure clinical guidelines[J]. Am J Gastroenterol, 2022, 117( 2): 225- 252. DOI: 10.14309/ajg.0000000000001595. [3] MOREAU R, JALAN R, GINES P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis[J]. Gastroenterology, 2013, 144( 7): 1426- 1437. DOI: 10.1053/j.gastro.2013.02.042. [4] SARIN SK, CHOUDHURY A, SHARMA MK, et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific Association for the Study of the Liver(APASL): An update[J]. Hepatol Int, 2019, 13( 4): 353- 390. DOI: 10.1007/s12072-019-09946-3. [5] LI JQ, LIANG X, YOU SL, et al. Development and validation of a new prognostic score for hepatitis B virus-related acute-on-chronic liver failure[J]. J Hepatol, 2021, 75( 5): 1104- 1115. DOI: 10.1016/j.jhep.2021.05.026. [6] PUGH RN, MURRAY-LYON IM, DAWSON JL, et al. Transection of the oesophagus for bleeding oesophageal varices[J]. Br J Surg, 1973, 60( 8): 646- 649. DOI: 10.1002/bjs.1800600817. [7] MALINCHOC M, KAMATH PS, GORDON FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts[J]. Hepatology, 2000, 31( 4): 864- 871. DOI: 10.1053/he.2000.5852. [8] BIGGINS SW, KIM WR, TERRAULT NA, et al. Evidence-based incorporation of serum sodium concentration into MELD[J]. Gastroenterology, 2006, 130( 6): 1652- 1660. DOI: 10.1053/j.gastro.2006.02.010. [9] LUCA A, ANGERMAYR B, BERTOLINI G, et al. An integrated MELD model including serum sodium and age improves the prediction of early mortality in patients with cirrhosis[J]. Liver Transpl, 2007, 13( 8): 1174- 1180. DOI: 10.1002/lt.21197. [10] Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Guideline for diagnosis and treatment of liver failure(2024 version)[J]. J Clin Hepatol, 2024, 40( 12): 2371- 2387. DOI: 10.12449/JCH241-206.中华医学会感染病学分会肝衰竭与人工肝学组, 中华医学会肝病学分会重型肝病与人工肝学组. 肝衰竭诊治指南(2024年版)[J]. 临床肝胆病杂志, 2024, 40( 12): 2371- 2387. DOI: 10.12449/JCH241206. [11] Chinese Society of Hepatology, Chinese Medical Association. Chinese guidelines on the management of liver cirrhosis[J]. J Clin Hepatol, 2019, 35( 11): 2408- 2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006.中华医学会肝病学分会. 肝硬化诊治指南[J]. 临床肝胆病杂志, 2019, 35( 11): 2408- 2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006. [12] WU TZ, LI J, SHAO L, et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure[J]. Gut, 2018, 67( 12): 2181- 2191. DOI: 10.1136/gutjnl-2017-314641. [13] JALAN R, YURDAYDIN C, BAJAJ JS, et al. Toward an improved definition of acute-on-chronic liver failure[J]. Gastroenterology, 2014, 147( 1): 4- 10. DOI: 10.1053/j.gastro.2014.05.005. [14] TREBICKA J. Predisposing factors in acute-on-chronic liver failure[J]. Semin Liver Dis, 2016, 36( 2): 167- 173. DOI: 10.1055/s-0036-1583195. [15] BAJAJ JS, O’LEARY JG, REDDY KR, et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures[J]. Hepatology, 2014, 60( 1): 250- 256. DOI: 10.1002/hep.27077. [16] MARTIN-MATEOS R, ALVAREZ-MON M, ALBILLOS A. Dysfunctional immune response in acute-on-chronic liver failure: It takes two to tango[J]. Front Immunol, 2019, 10: 973. DOI: 10.3389/fimmu.2019.00973. [17] WU W, SUN SS, WANG YJ, et al. Circulating neutrophil dysfunction in HBV-related acute-on-chronic liver failure[J]. Front Immunol, 2021, 12: 620365. DOI: 10.3389/fimmu.2021.620365. [18] LEMMER P, POSPIECH JC, CANBAY A. Liver failure-future challenges and remaining questions[J]. Ann Transl Med, 2021, 9( 8): 734. DOI: 10.21037/atm-20-4968. [19] KIM A, NIU BL, WORETA T, et al. Clinical considerations of coagulopathy in acute liver failure[J]. J Clin Transl Hepatol, 2020, 8( 4): 407- 413. DOI: 10.14218/JCTH.2020.00058. [20] van den BOOM BP, LISMAN T. Pathophysiology and management of bleeding and thrombosis in patients with liver disease[J]. Int J Lab Hematol, 2022, 44( Suppl 1): 79- 88. DOI: 10.1111/ijlh.13856. [21] LAI M, XU MM, WANG X, et al. Prognostic evaluation of liver transplantation for acute-on-chronic liver failure[J]. Organ Transplant, 2025, 16( 3): 482- 488. DOI: 10.12464/j.issn.1674-7445.2025002.赖曼, 徐曼曼, 王鑫, 等. 慢加急性肝衰竭肝移植预后评估[J]. 器官移植, 2025, 16( 3): 482- 488. DOI: 10.12464/j.issn.1674-7445.2025002. -



 PDF下载 ( 2338 KB)
PDF下载 ( 2338 KB)

 下载:
下载: