人工智能在代谢相关脂肪性肝病中的应用
DOI: 10.12449/JCH251103
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:孙超负责查阅文献,撰写论文;范建高负责修改文章并最后定稿。
-
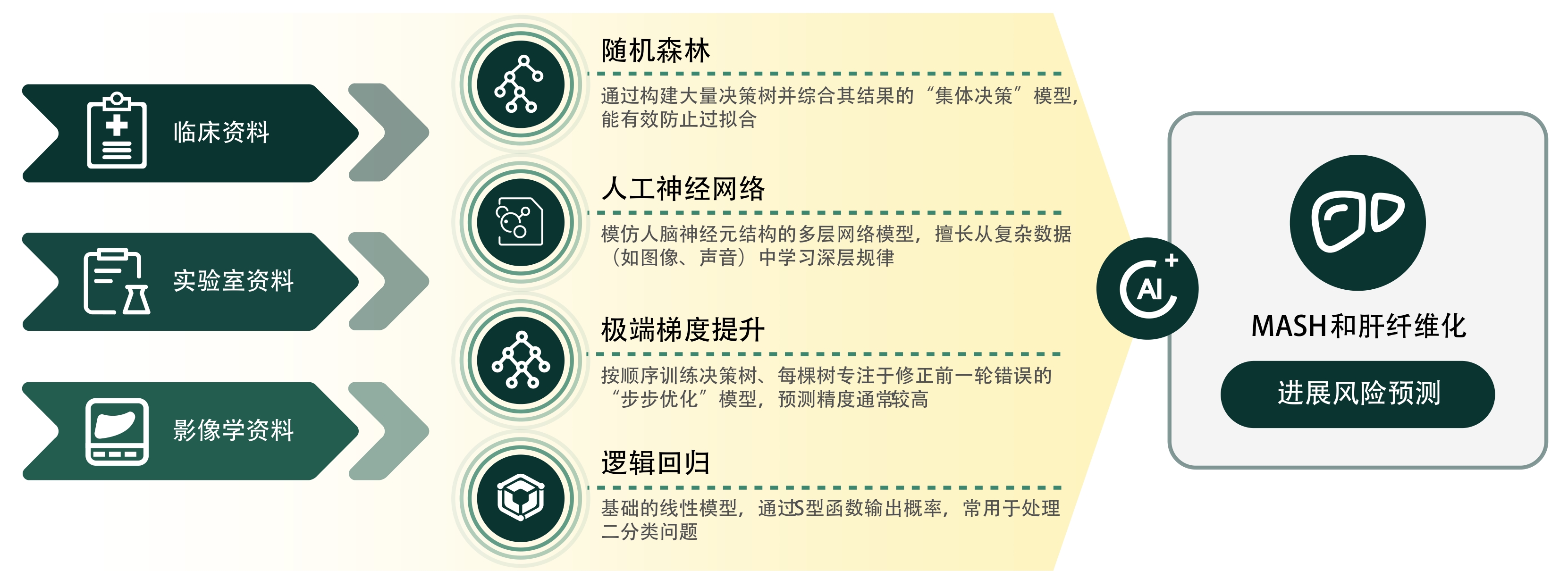
摘要: 随着肥胖和代谢综合征的流行,代谢相关脂肪性肝病(MAFLD)已成为我国乃至全球最常见的慢性肝病。传统的诊断和监测方法依赖于肝活检、影像学和血清学标志物,但存在侵入性、成本高和灵敏度不足等局限性。近年来,人工智能(AI)技术在医学领域的快速发展为MAFLD的诊疗提供了新思路。本文探讨了AI技术在MAFLD疾病诊断模型、疾病进展预测和数字治疗等领域的应用,旨在为MAFLD的诊断和管理提供借鉴。Abstract: With the prevalence of obesity and metabolic syndrome, metabolic associated fatty liver disease (MAFLD) has become one of the most common chronic liver diseases in China and globally. Traditional diagnostic and monitoring methods rely on liver biopsy, imaging techniques, and serological markers, and their application is limited by invasiveness, high costs, and insufficient sensitivity. In recent years, the rapid development of artificial intelligence (AI) technology in the medical field has provided new ideas for the diagnosis and treatment of MAFLD. This article explores the application of AI technology in areas such as models for the diagnosis of MAFLD, the prediction of disease progression, and digital therapeutics, in order to provide a reference for the diagnosis and management of MAFLD.
-
[1] ZHAO J, LIU L, CAO YY, et al. MAFLD as part of systemic metabolic dysregulation[J]. Hepatol Int, 2024, 18( Suppl 2): 834- 847. DOI: 10.1007/s12072-024-10660-y. [2] LOU TW, YANG RX, FAN JG. The global burden of fatty liver disease: The major impact of China[J]. Hepatobiliary Surg Nutr, 2024, 13( 1): 119- 123. DOI: 10.21037/hbsn-23-556. [3] YOUNOSSI ZM, KALLIGEROS M, HENRY L. Epidemiology of metabolic dysfunction-associated steatotic liver disease[J]. Clin Mol Hepatol, 2025, 31( Suppl): S32- S50. DOI: 10.3350/cmh.2024.0431. [4] NAM D, CHAPIRO J, PARADIS V, et al. Artificial intelligence in liver diseases: Improving diagnostics, prognostics and response prediction[J]. JHEP Rep, 2022, 4( 4): 100443. DOI: 10.1016/j.jhepr.2022.100443. [5] CADDEO A, ROMEO S. Precision medicine and nucleotide-based therapeutics to treat steatotic liver disease[J]. Clin Mol Hepatol, 2025, 31( Suppl): S76- S93. DOI: 10.3350/cmh.2024.0438. [6] RATZIU V, FRIEDMAN SL. Why do so many nonalcoholic steatohepatitis trials fail?[J]. Gastroenterology, 2023, 165( 1): 5- 10. DOI: 10.1053/j.gastro.2020.05.046. [7] ABDELHAMEED F, KITE C, LAGOJDA L, et al. Non-invasive scores and serum biomarkers for fatty liver in the era of metabolic dysfunction-associated steatotic liver disease(MASLD): A comprehensive review from NAFLD to MAFLD and MASLD[J]. Curr Obes Rep, 2024, 13( 3): 510- 531. DOI: 10.1007/s13679-024-00574-z. [8] MIAO L, TARGHER G, BYRNE CD, et al. Current status and future trends of the global burden of MASLD[J]. Trends Endocrinol Metab, 2024, 35( 8): 697- 707. DOI: 10.1016/j.tem.2024.02.007. [9] DENG JL, JI WD, LIU HZ, et al. Development and validation of a machine learning-based framework for assessing metabolic-associated fatty liver disease risk[J]. BMC Public Health, 2024, 24( 1): 2545. DOI: 10.1186/s12889-024-19882-z. [10] HUANG GQ, JIN QK, MAO YS. Predicting the 5-year risk of nonalcoholic fatty liver disease using machine learning models: Prospective cohort study[J]. J Med Internet Res, 2023, 25: e46891. DOI: 10.2196/46891. [11] DESTREMPES F, GESNIK M, CHAYER B, et al. Quantitative ultrasound, elastography, and machine learning for assessment of steatosis, inflammation, and fibrosis in chronic liver disease[J]. PLoS One, 2022, 17( 1): e0262291. DOI: 10.1371/journal.pone.0262291. [12] ALSHAGATHRH FM, HOUSEH MS. Artificial intelligence for detecting and quantifying fatty liver in ultrasound images: A systematic review[J]. Bioengineering, 2022, 9( 12): 748. DOI: 10.3390/bioengineering9120748. [13] PICKHARDT PJ, BLAKE GM, GRAFFY PM, et al. Liver steatosis categorization on contrast-enhanced CT using a fully automated deep learning volumetric segmentation tool: Evaluation in 1204 healthy adults using unenhanced CT as a reference standard[J]. AJR Am J Roentgenol, 2021, 217( 2): 359- 367. DOI: 10.2214/AJR.20.24415. [14] CHOI KJ, JANG JK, LEE SS, et al. Development and validation of a deep learning system for staging liver fibrosis by using contrast agent-enhanced CT images in the liver[J]. Radiology, 2018, 289( 3): 688- 697. DOI: 10.1148/radiol.2018180763. [15] YASAKA K, AKAI H, KUNIMATSU A, et al. Liver fibrosis: Deep convolutional neural network for staging by using gadoxetic acid-enhanced hepatobiliary phase MR images[J]. Radiology, 2018, 287( 1): 146- 155. DOI: 10.1148/radiol.2017171928. [16] ZARELLA MD, BOWMAN D, AEFFNER F, et al. A practical guide to whole slide imaging: A white paper from the digital pathology association[J]. Arch Pathol Lab Med, 2019, 143( 2): 222- 234. DOI: 10.5858/arpa.2018-0343-RA. [17] TAYLOR-WEINER A, POKKALLA H, HAN L, et al. A machine learning approach enables quantitative measurement of liver histology and disease monitoring in NASH[J]. Hepatology, 2021, 74( 1): 133- 147. DOI: 10.1002/hep.31750. [18] ASTBURY S, GROVE JI, DORWARD DA, et al. Reliable computational quantification of liver fibrosis is compromised by inherent staining variation[J]. J Pathol Clin Res, 2021, 7( 5): 471- 481. DOI: 10.1002/cjp2.227. [19] LEOW WQ, BEDOSSA P, LIU F, et al. An improved qFibrosis algorithm for precise screening and enrollment into non-alcoholic steatohepatitis(NASH) clinical trials[J]. Diagnostics, 2020, 10( 9): 643. DOI: 10.3390/diagnostics10090643. [20] BOSCH J, CHUNG C, CARRASCO-ZEVALLOS OM, et al. A machine learning approach to liver histological evaluation predicts clinically significant portal hypertension in NASH cirrhosis[J]. Hepatology, 2021, 74( 6): 3146- 3160. DOI: 10.1002/hep.32087. [21] RATZIU V, HOMPESCH M, PETITJEAN M, et al. Artificial intelligence-assisted digital pathology for non-alcoholic steatohepatitis: Current status and future directions[J]. J Hepatol, 2024, 80( 2): 335- 351. DOI: 10.1016/j.jhep.2023.10.015. [22] NJEI B, OSTA E, NJEI N, et al. An explainable machine learning model for prediction of high-risk nonalcoholic steatohepatitis[J]. Sci Rep, 2024, 14( 1): 8589. DOI: 10.1038/s41598-024-59183-4. [23] MUNK LAURIDSEN M, RAVNSKJAER K, GLUUD LL, et al. Disease classification, diagnostic challenges, and evolving clinical trial design in MASLD[J]. J Clin Invest, 2025, 135( 10): e189953. DOI: 10.1172/JCI189953. [24] CHANG D, TRUONG E, MENA EA, et al. Machine learning models are superior to noninvasive tests in identifying clinically significant stages of NAFLD and NAFLD-related cirrhosis[J]. Hepatology, 2023, 77( 2): 546- 557. DOI: 10.1002/hep.32655. [25] KWON OY, CHOI JY, JANG Y. The effectiveness of eHealth interventions on lifestyle modification in patients with nonalcoholic fatty liver disease: Systematic review and meta-analysis[J]. J Med Internet Res, 2023, 25: e37487. DOI: 10.2196/37487. [26] TINCOPA MA, PATEL N, SHAHAB A, et al. Implementation of a randomized mobile-technology lifestyle program in individuals with nonalcoholic fatty liver disease[J]. Sci Rep, 2024, 14( 1): 7452. DOI: 10.1038/s41598-024-57722-7. [27] TINCOPA MA, LYDEN A, WONG J, et al. Impact of a pilot structured mobile technology based lifestyle intervention for patients with nonalcoholic fatty liver disease[J]. Dig Dis Sci, 2022, 67( 2): 481- 491. DOI: 10.1007/s10620-021-06922-6. [28] SATO M, AKAMATSU M, SHIMA T, et al. Impact of a novel digital therapeutics system on nonalcoholic steatohepatitis: The NASH app clinical trial[J]. Am J Gastroenterol, 2023, 118( 8): 1365- 1372. DOI: 10.14309/ajg.0000000000002143. [29] KWON OY, LEE MK, LEE HW, et al. Mobile app-based lifestyle coaching intervention for patients with nonalcoholic fatty liver disease: Randomized controlled trial[J]. J Med Internet Res, 2024, 26: e49839. DOI: 10.2196/49839. -



 PDF下载 ( 648 KB)
PDF下载 ( 648 KB)

 下载:
下载:


