人工智能在肝衰竭预警及预后体系中的应用与挑战
DOI: 10.12449/JCH251104
Application and challenges of artificial intelligence in prediction and prognosis systems for liver failure
-
摘要: 肝衰竭是由多种因素引起的严重肝损害,导致肝脏合成、解毒、代谢和生物转化功能严重障碍或失代偿,出现以黄疸、凝血功能障碍、肝肾综合征、肝性脑病及腹水等为主要表现的一组临床综合征,早期精准预后预测对改善患者临床结局至关重要。近年来,基于人工智能(AI)的预警预测模型正逐步改变传统诊疗模式。本文系统综述了利用机器学习构建的预警及预后模型在急性肝衰竭及慢加急性肝衰竭中的应用进展,相关模型在风险分层与预后预测方面展现出良好性能。随着技术的不断发展,AI有望为肝衰竭的早期干预和精准治疗提供新机遇,从而显著改善患者预后。Abstract: Liver failure is a severe liver injury caused by multiple factors, leading to significant impairment or decompensation of the liver’s synthetic, detoxification, metabolic, and biotransformation functions, and it is a group of clinical syndromes with the main manifestations of jaundice, coagulation disorder, hepatorenal syndrome, hepatic encephalopathy, and ascites. Early and accurate prognostic prediction is crucial for improving the clinical outcome of patients. In recent years, artificial intelligence (AI)-based early warning and prediction models are gradually transforming the traditional diagnostic and therapeutic approaches. This article systematically reviews the advances in the application of machine learning-based early warning and prediction models in acute liver failure and acute-on-chronic liver failure, and related models have shown good performance in risk stratification and prognosis prediction. With the continuous development of related technologies, AI is expected to provide new opportunities for the early intervention and precise treatment of liver failure, thereby significantly improving the prognosis of patients.
-
Key words:
- Liver Failure /
- Artificial Intelligence /
- Machine Learning /
- Early Diagnosis /
- Prognosis
-
表 1 AI在疾病预测及预警中的应用
Table 1. The application of AI in disease prediction and early warning
算法类型 原理 应用场景 监督学习 线性回归 所有数据点的最佳拟合线 连续型(数值)结果预测 逻辑回归 采用逻辑函数对分类因变量建模 临床结局预测;疾病类别划分 Cox回归 通过特征预测个体随时间变化的事件风险 风险随时间变化的事件预测 支持向量机 通过核技巧实现高维特征的非线性分类 风险预测;疾病分类;图像识别 K近邻算法 基于特征向量间欧氏距离进行预测 连续/分类结局预测 决策树 树状模型进行是/否决策和结果预测 临床事件的预测;疾病类别划分 随机森林 多棵决策树组成的集成模型 临床事件的预测;疾病类别划分 梯度提升 较弱预测模型的分阶段集成 临床事件的预测;疾病类别划分 分类与回归树 通过递归分割数据生成规则 临床事件的预测;疾病类别划分 贝叶斯网络 用带概率的有向图描述变量关系 临床事件的预测;疾病类别划分 无监督学习 K均值聚类 基于欧氏距离将无标签数据分组 群体内的聚类识别 潜在类别模型 识别潜在亚型,辅助分群 辅助分群与预后判断 主成分分析 基于正交变换的无监督线性降维方法 降维可视化;特征提取;消除共线性 层次聚类分析 合并最相似的数据点生成一棵展现数据层次的分类树 数据内在层次结构分析 神经网络与深度学习 人工神经网络 模拟神经元信号传递的计算结构 多模态数据融合分析 卷积神经网络 仿生物视觉皮层连接模式 图像识别;自然语言处理 循环神经网络 沿时间序列构建有向图 手写/语音识别;临床事件的预测 长短期记忆网络 特殊的循环神经网络,通过门控机制解决长期依赖 动态预警;多参数监护 Transformer 基于自注意力机制,通过位置编码保留时序信息 多模态融合;长序列预测 表 2 基于AI的肝衰竭预警及预后常用模型总结
Table 2. Summary of commonly used AI-based models for liver failure early warning and prognosis
模型 应用人群 评价指标 评分公式 临床结局 机器学习算法 ALF预后评分模型 KCC[20] APAP相关暴发性肝衰
竭、非APAP
相关暴发性肝衰竭动脉血pH、肌酐、HE
等级、PT、年龄、TBil、
病因/ 住院期间
病死率逻辑回归 MELD[21] 终末期肝病 TBil、INR、肌酐、病因 3.78×lnTBil(mg/dL)+11.2×
ln(INR)+9.6×ln肌酐(mg/dL)
+6.43×病因90天病死率 Cox比例风险
回归、逻辑回归ALFSG-PI[22] APAP诱发ALF、
非APAP诱发ALFHE等级、病因、血管
升压药的使用、TBil、
INR2.67-0.95×HE+1.56×病因
-1.25×血管升压药使用
-0.70×lnTBil(mg/dL)-1.35
×lnINR21天病死率 逻辑回归 DIALF-5[23] 非APAP诱发ALF INR、HE等级、血管升
压药的使用、N-乙酰
半胱氨酸和人工肝支
持系统使用/ 21天病死率 Cox比例风险
回归ACLF预后评分模型 COSSH-ACLF Ⅱ[24] 慢性肝病急性失代偿
(不论有无肝硬化)INR、HE等级、
中性粒细胞、TBil、
血尿素、年龄1.649×ln(INR)+0.457×HE
评分+0.425×ln(中性粒细胞)
+0.396×ln(TBil)+0.576×
ln(血尿素)+0.033×年龄28/90天病
死率竞争风险回归、
LASSO回归COSSH-ACLF[10] 慢性肝病急性失代偿
(不论有无肝硬化)HBV-SOFA评分、
INR、TBil、年龄0.741×INR+0.523×HBV-
SOFA+0.026×年龄+0.003×
TBil(μmol/L)28/90天病
死率Cox比例风险
回归CLIF-C ACLF[27] 肝硬化急性
失代偿性CLIF-OF评分、年龄、
白细胞10×0.33×CLIF-OF+0.04×v年龄+0.63×ln(白细胞)-2 28/90天病
死率逻辑回归、
竞争风险回归CLIF-C MET[28] 肝硬化急性
失代偿性年龄、4-羟基-3-甲氧
基苯乙二醇硫酸盐、
己酰肉碱、D-半乳糖
醛酸[0.023 96×年龄+0.329 81×
log2(4-羟基-3-甲氧基苯乙二醇
硫酸盐)+0.456 02×log2(己酰
肉碱)+0.272 26×log2(D-半乳
糖醛酸)-18.156 1]/0.096 57/14/28/90天
病死率Cox比例风险
回归、竞争风险
回归CLIF-SIG[29] 肝硬化急性失代偿性 28个最具区分度的基
因表达水平/ 系统性炎症严
重程度、28天
和90天病死率LASSO回归 APASL-AARC[17] 慢性肝病急性恶化 TBil、肌酐、INR、HE
等级、血乳酸/ 28天病死率 Cox比例风险
回归NACSELD-ACLF[12] 肝硬化急性失代偿性 HE等级、肾脏替代治
疗、机械通气、平均动
脉压/ ≥2个器官衰竭
者30天病死率逻辑回归 ACLF预警评分模型 COSSH-onset-ACLF[26] 慢性肝病急性失代偿
(不论有无肝硬化)ALT、TBil、INR、
铁蛋白0.101×ln(ALT)+0.819×
ln(TBil)+2.820×ln(INR)
+0.016×ln(铁蛋白)ACLF发生率 LASSO-Cox
回归注:HBV-SOFA,乙型肝炎相关序贯器官衰竭评估评分;/,无具体公式,根据评价指标判断。
表 3 基于AI的肝衰竭预警及预后标志物总结
Table 3. Summary of AI-based biomarkers for liver failure early warning and prognosis
组学类型 生物标志物 来源 目标疾病 预后影响 机器学习算法 基因组学 rs3129859-C、HLA-
DRB1*12∶02[46]全血DNA HBV-ACLF 携带rs3129859-C或HLA-DRB1*12∶02
风险等位基因,则凝血差、易出现腹
水,且28天病死率更高逻辑回归、线性回归 转录组学 SEMA6B、THBS1、
MERTK、PPARG、
ETS2、VSIG4PBMC HBV-ACLF SEMA6B、THBS1、MERTK、PPARG、
ETS2和VSIG4高表达标志着剧烈的
炎症反应和高短期病死率层次聚类和主成分分析 COL4A2[32] 肝组织 ALF COL4A2高表达提示疾病更严重,短
期预后差LASSO回归、支持向量机 VCAN、CXCR2[52] PBMC/肝组织 HBV-ACLF 早期VCAN+CD14+单核细胞驱动
炎症风暴;后期CXCR2+中性粒
细胞导致免疫耗竭及恶化UMAP、RNA 速率、
SCENIC、CellChat、
Cox比例风险回归Annexin A1[53] PBMC/
肝组织ALF/ACLF Annexin A1高表达提示机体抗炎
代偿能力强,预后较好UMAP、SCENIC、CellChat 蛋白质组学 APOC3、HRG、TF、
KLKB蛋白[54]血浆 HBV-ACLF APOC3、HRG、TF和KLKB1在
ACLF中显著下调,与高病死率相关,
可用于慢性乙型肝炎区分逻辑回归、Cox比例风险
回归、主成分分析代谢组学 哌可酸、N-乙酰天门冬
酰谷氨酸、3-脲基丙酸、
γ-羧乙基羟色胺[55]血浆 HBV-ACLF 哌可酸、N-乙酰天门冬酰谷氨酸、
3-脲基丙酸的水平升高,γ-羧乙基羟
色胺的水平降低预示ACLF的
发生和不良预后随机森林、逻辑回归 FGF21、C6∶0-和C8∶0-
肉碱[56]血浆 ACLF FGF21、C6∶0-和C8∶0-肉碱高表达提
示高短期病死率偏最小二乘判别分析 微生物组学 基因丰富度、宏基因组
物种丰富度[57]粪便 ACLF 基因丰富度、MGS丰富度显著下
降提示疾病更严重,短期预后差逻辑回归、随机森林 注:PBMC,外周血单个核细胞;SEMA6B,信号素6B;THBS1,血小板反应蛋白1;MERTK,原癌基因酪氨酸蛋白激酶MER;PPARG,过氧化物酶体增殖物激活受体γ;ETS2,ETS原癌基因2型转型录因子;VSIG4,含V-set和免疫球蛋白结构域蛋白4;COL4A2,Ⅳ型胶原蛋白α2链;VCAN,蛋白聚糖Versican;CXCR2,C-X-C基序趋化因子受体2;Annexin A1,膜联蛋白A1;FGF21,成纤维细胞生长因子21。
-
[1] Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Guideline for diagnosis and treatment of liver failure(2024 version)[J]. J Clin Hepatol, 2024, 40( 12): 2371- 2387. DOI: 10.12449/JCH241206.中华医学会感染病学分会肝衰竭与人工肝学组, 中华医学会肝病学分会重型肝病与人工肝学组. 肝衰竭诊治指南(2024年版)[J]. 临床肝胆病杂志, 2024, 40( 12): 2371- 2387. DOI: 10.12449/JCH241206. [2] MAIWALL R, KULKARNI AV, ARAB JP, et al. Acute liver failure[J]. Lancet, 2024, 404( 10454): 789- 802. DOI: 10.1016/S0140-6736(24)00693-7. [3] ARROYO V, MOREAU R, JALAN R. Acute-on-chronic liver failure[J]. N Engl J Med, 2020, 382( 22): 2137- 2145. DOI: 10.1056/nejmra1914900. [4] LUO JJ, LI JQ, LI P, et al. Acute-on-chronic liver failure: Far to go-a review[J]. Crit Care, 2023, 27( 1): 259. DOI: 10.1186/s13054-023-04540-4. [5] KULKARNI AV, SARIN SK. Acute-on-chronic liver failure–steps towards harmonization of the definition![J]. J Hepatol, 2024, 81( 2): 360- 366. DOI: 10.1016/j.jhep.2024.03.036. [6] ZACCHERINI G, WEISS E, MOREAU R. Acute-on-chronic liver failure: Definitions, pathophysiology and principles of treatment[J]. JHEP Rep, 2021, 3( 1): 100176. DOI: 10.1016/j.jhepr.2020.100176. [7] SIDEY-GIBBONS JAM, SIDEY-GIBBONS CJ. Machine learning in medicine: A practical introduction[J]. BMC Med Res Methodol, 2019, 19( 1): 64. DOI: 10.1186/s12874-019-0681-4. [8] JIANG F, JIANG Y, ZHI H, et al. Artificial intelligence in healthcare: Past, present and future[J]. Stroke Vasc Neurol, 2017, 2( 4): 230- 243. DOI: 10.1136/svn-2017-000101. [9] AHN JC, CONNELL A, SIMONETTO DA, et al. Application of artificial intelligence for the diagnosis and treatment of liver diseases[J]. Hepatology, 2021, 73( 6): 2546- 2563. DOI: 10.1002/hep.31603. [10] WU TZ, LI J, SHAO L, et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure[J]. Gut, 2018, 67( 12): 2181- 2191. DOI: 10.1136/gutjnl-2017-314641. [11] MOREAU R, JALAN R, GINES P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis[J]. Gastroenterology, 2013, 144( 7): 1426- 1437, 1437. e1- 9. DOI: 10.1053/j.gastro.2013.02.042. [12] O’LEARY JG, REDDY KR, GARCIA-TSAO G, et al. NACSELD acute-on-chronic liver failure(NACSELD-ACLF) score predicts 30-day survival in hospitalized patients with cirrhosis[J]. Hepatology, 2018, 67( 6): 2367- 2374. DOI: 10.1002/hep.29773. [13] SARIN SK, KUMAR A, ALMEIDA JA, et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific Association for the study of the liver(APASL)[J]. Hepatol Int, 2009, 3( 1): 269- 282. DOI: 10.1007/s12072-008-9106-x. [14] European Association for the Study of the Liver. EASL clinical practice guidelines on acute-on-chronic liver failure[J]. J Hepatol, 2023, 79( 2): 461- 491. DOI: 10.1016/j.jhep.2023.04.021. [15] KARVELLAS CJ, BAJAJ JS, KAMATH PS, et al. AASLD practice guidance on acute-on-chronic liver failure and the management of critically ill patients with cirrhosis[J]. Hepatology, 2024, 79( 6): 1463- 1502. DOI: 10.1097/HEP.0000000000000671. [16] LUO JJ, HU MQ, FENG TT, et al. Performance of the China-CLIF framework in acute-on-chronic liver failure: A multicohort study across all aetiologies[J]. Gut, 2025. DOI: 10.1136/gutjnl-2025-335651.[ Epub ahead of print] [17] CHOUDHURY A, JINDAL A, MAIWALL R, et al. Liver failure determines the outcome in patients of acute-on-chronic liver failure(ACLF): Comparison of APASL ACLF research consortium(AARC) and CLIF-SOFA models[J]. Hepatol Int, 2017, 11( 5): 461- 471. DOI: 10.1007/s12072-017-9816-z. [18] RAJKOMAR A, DEAN J, KOHANE I. Machine learning in medicine[J]. N Engl J Med, 2019, 380( 14): 1347- 1358. DOI: 10.1056/nejmra1814259. [19] SILVEY S, KAMATH PS, GEORGE J, et al. Enhancement of inpatient mortality prognostication with machine learning in a prospective global cohort of patients with cirrhosis with external validation[J]. Gastroenterology, 2025. DOI: 10.1053/j.gastro.2025.07.015.[ Online ahead of print] [20] ANAND AC, NIGHTINGALE P, NEUBERGER JM. Early indicators of prognosis in fulminant hepatic failure: An assessment of the King’s criteria[J]. J Hepatol, 1997, 26( 1): 62- 68. DOI: 10.1016/s0168-8278(97)80010-4. [21] KAMATH PS, WIESNER RH, MALINCHOC M, et al. A model to predict survival in patients with end-stage liver disease[J]. Hepatology, 2001, 33( 2): 464- 470. DOI: 10.1053/jhep.2001.22172. [22] KOCH DG, TILLMAN H, DURKALSKI V, et al. Development of a model to predict transplant-free survival of patients with acute liver failure[J]. Clin Gastroenterol Hepatol, 2016, 14( 8): 1199- 1206. e 2. DOI: 10.1016/j.cgh.2016.03.046. [23] HAN L, HUANG A, CHEN JJ, et al. Clinical characteristics and prognosis of non-APAP drug-induced acute liver failure: A large multicenter cohort study[J]. Hepatol Int, 2024, 18( 1): 225- 237. DOI: 10.1007/s12072-023-10541-w. [24] LI JQ, LIANG X, YOU SL, et al. Development and validation of a new prognostic score for hepatitis B virus-related acute-on-chronic liver failure[J]. J Hepatol, 2021, 75( 5): 1104- 1115. DOI: 10.1016/j.jhep.2021.05.026. [25] HU MQ, LUO JJ, WU Y, et al. Integrating prior decompensation into ACLF definition to enhance clinical management[J]. Hepatol Int, 2025. DOI: 10.1007/s12072-025-10805-7.[ Online ahead of print] [26] LUO JJ, LIANG X, XIN JJ, et al. Predicting the onset of hepatitis B virus-related acute-on-chronic liver failure[J]. Clin Gastroenterol Hepatol, 2023, 21( 3): 681- 693. DOI: 10.1016/j.cgh.2022.03.016. [27] JALAN R, SALIBA F, PAVESI M, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure[J]. J Hepatol, 2014, 61( 5): 1038- 1047. DOI: 10.1016/j.jhep.2014.06.012. [28] WEISS E, DE LA PEÑA-RAMIREZ C, AGUILAR F, et al. Sympathetic nervous activation, mitochondrial dysfunction and outcome in acutely decompensated cirrhosis: The metabolomic prognostic models(CLIF-C MET)[J]. Gut, 2023, 72( 8): 1581- 1591. DOI: 10.1136/gutjnl-2022-328708. [29] TREBICKA J, AGUILAR F, QUEIROZ FARIAS A, et al. Gene score to quantify systemic inflammation in patients with acutely decompensated cirrhosis[J]. Gut, 2025, 74( 8): 1293- 1307. DOI: 10.1136/gutjnl-2024-333876. [30] SPEISER JL, LEE WM, KARVELLAS CJ. Predicting outcome on admission and post-admission for acetaminophen-induced acute liver failure using classification and regression tree models[J]. PLoS One, 2015, 10( 4): e0122929. DOI: 10.1371/journal.pone.0122929. [31] SPEISER JL, KARVELLAS CJ, WOLF BJ, et al. Predicting daily outcomes in acetaminophen-induced acute liver failure patients with machine learning techniques[J]. Comput Methods Programs Biomed, 2019, 175: 111- 120. DOI: 10.1016/j.cmpb.2019.04.012. [32] YUAN MQ, YAO LC, HU X, et al. Identification of effective diagnostic biomarker and immune cell infiltration characteristics in acute liver failure by integrating bioinformatics analysis and machine-learning strategies[J]. Front Genet, 2022, 13: 1004912. DOI: 10.3389/fgene.2022.1004912. [33] PAPPADA S, SATHELLY B, SCHMIEDER J, et al. An artificial neural network approach to diagnose and predict liver dysfunction and failure in the critical care setting[J]. Hippokratia, 2024, 28( 1): 1- 10. [34] DONG R, LUO ZH, XUE H, et al. Development and validation of an explainable machine learning model for warning of hepatitis E virus-related acute liver failure[J]. Liver Int, 2025, 45( 6): e70129. DOI: 10.1111/liv.70129. [35] SHI KQ, ZHOU YY, YAN HD, et al. Classification and regression tree analysis of acute-on-chronic hepatitis B liver failure: Seeing the forest for the trees[J]. J Viral Hepat, 2017, 24( 2): 132- 140. DOI: 10.1111/jvh.12617. [36] ZHENG MH, SHI KQ, LIN XF, et al. A model to predict 3-month mortality risk of acute-on-chronic hepatitis B liver failure using artificial neural network[J]. J Viral Hepat, 2013, 20( 4): 248- 255. DOI: 10.1111/j.1365-2893.2012.01647.x. [37] HOU YX, ZHANG QQ, GAO FY, et al. Artificial neural network-based models used for predicting 28- and 90-day mortality of patients with hepatitis B-associated acute-on-chronic liver failure[J]. BMC Gastroenterol, 2020, 20( 1): 75. DOI: 10.1186/s12876-020-01191-5. [38] MUSUNURI B, SHETTY S, SHETTY DK, et al. Acute-on-chronic liver failure mortality prediction using an artificial neural network[J]. Eng Sci, 2021, 15: 187- 196. DOI: 10.30919/es8d515 [39] GARCIA MS, AGARWAL B, MOOKERJEE RP, et al. An accurate data preparation approach for the prediction of mortality in ACLF patients using the CANONIC dataset[J]. Annu Int Conf IEEE Eng Med Biol Soc, 2019, 2019: 1371- 1377. DOI: 10.1109/EMBC.2019.8857239. [40] VERMA N, CHOUDHURY A, SINGH V, et al. APASL-ACLF Research Consortium-Artificial Intelligence(AARC-AI) model precisely predicts outcomes in acute-on-chronic liver failure patients[J]. Liver Int, 2023, 43( 2): 442- 451. DOI: 10.1111/liv.15361. [41] QIU ST, ZHAO YM, HU JX, et al. Predicting the 28-day prognosis of acute-on-chronic liver failure patients based on machine learning[J]. Dig Liver Dis, 2024, 56( 12): 2095- 2102. DOI: 10.1016/j.dld.2024.06.029. [42] XU YT, ZHANG YQ, YANG ZJ, et al. Imbalanced and semi-supervised classification for prognosis of ACLF[J]. J Intell Fuzzy Syst, 2015, 28( 2): 737- 745. DOI: 10.5555/2729770.2729793. [43] XIE ZB, DING L, LI YZ. Computed tomography image features under convolutional neural network algorithm in analysis of inflammatory factor level and prognosis of patients with hepatitis B virus-associated acute-on-chronic liver failure[J]. J Healthc Eng, 2021, 2021: 2110612. DOI: 10.1155/2021/2110612. [44] VERMA N, GARG P, VALSAN A, et al. Identification of four novel acute-on-chronic liver failure clusters with distinct clinical trajectories and mortality using machine learning methods[J]. Aliment Pharmacol Ther, 2024, 60( 11-12): 1534- 1548. DOI: 10.1111/apt.18274. [45] LI P, LIANG X, LUO JJ, et al. Omics in acute-on-chronic liver failure[J]. Liver Int, 2025, 45( 3): e15634. DOI: 10.1111/liv.15634. [46] TAN WT, XIA J, DAN YJ, et al. Genome-wide association study identifies HLA-DR variants conferring risk of HBV-related acute-on-chronic liver failure[J]. Gut, 2018, 67( 4): 757- 766. DOI: 10.1136/gutjnl-2016-313035. [47] LI J, LIANG X, JIANG J, et al. PBMC transcriptomics identifies immune-metabolism disorder during the development of HBV-ACLF[J]. Gut, 2022, 71( 1): 163- 175. DOI: 10.1136/gutjnl-2020-323395. [48] HE LL, CAI Q, LIANG X, et al. ETS2 alleviates acute-on-chronic liver failure by suppressing excessive inflammation[J]. J Med Virol, 2023, 95( 4): e28710. DOI: 10.1002/jmv.28710. [49] LIANG X, LI P, JIANG J, et al. Transcriptomics unveils immune metabolic disruption and a novel biomarker of mortality in patients with HBV-related acute-on-chronic liver failure[J]. JHEP Rep, 2023, 5( 9): 100848. DOI: 10.1016/j.jhepr.2023.100848. [50] YANG H, CAI Q, XIN JJ, et al. SEMA6B induces macrophage-mediated inflammation and hepatocyte apoptosis in hepatitis B virus-related acute-on-chronic liver failure[J]. Theranostics, 2024, 14( 13): 5200- 5218. DOI: 10.7150/thno.97007. [51] HASSAN HM, LIANG X, XIN JJ, et al. Thrombospondin 1 enhances systemic inflammation and disease severity in acute-on-chronic liver failure[J]. BMC Med, 2024, 22( 1): 95. DOI: 10.1186/s12916-024-03318-x. [52] LIANG X, LUO JJ, ZHOU Q, et al. Single-cell multimodal analysis reveals the dynamic immunopathogenesis of HBV-ACLF progression[J]. Gut, 2025. DOI: 10.1136/gutjnl-2024-333308.[ Epub ahead of print] [53] YU X, TIAN W, BAO X, et al. Dissecting the liver inflammation ecosystem identifies annexin A1 as a pro-resolving target for liver failure[J]. Hepatology, 2025. DOI: 10.1097/HEP.0000000000001427.[ Epub ahead of print] [54] SUN ZY, LIU XL, WU DX, et al. Circulating proteomic panels for diagnosis and risk stratification of acute-on-chronic liver failure in patients with viral hepatitis B[J]. Theranostics, 2019, 9( 4): 1200- 1214. DOI: 10.7150/thno.31991. [55] ZHANG Y, TAN WT, WANG XB, et al. Metabolic biomarkers significantly enhance the prediction of HBV-related ACLF occurrence and outcomes[J]. J Hepatol, 2023, 79( 5): 1159- 1171. DOI: 10.1016/j.jhep.2023.07.011. [56] ZHANG IW, CURTO A, LÓPEZ-VICARIO C, et al. Mitochondrial dysfunction governs immunometabolism in leukocytes of patients with acute-on-chronic liver failure[J]. J Hepatol, 2022, 76( 1): 93- 106. DOI: 10.1016/j.jhep.2021.08.009. [57] SOLÉ C, GUILLY S, SILVA K DA, et al. Alterations in gut microbiome in cirrhosis as assessed by quantitative metagenomics: Relationship with acute-on-chronic liver failure and prognosis[J]. Gastroenterology, 2021, 160( 1): 206- 218. e 13. DOI: 10.1053/j.gastro.2020.08.054. [58] WIEST IC, WOLF F, LEßMANN ME, et al. LLM-AIx: An open source pipeline for Information Extraction from unstructured medical text based on privacy preserving Large Language Models[J]. medRxiv, 2024. DOI: 10.1101/2024.09.02.24312917. [59] CLUSMANN J, BALAGUER-MONTERO M, BASSEGODA O, et al. The barriers for uptake of artificial intelligence in hepatology and how to overcome them[J]. J Hepatol, 2025. DOI: 10.1016/j.jhep.2025.07.003.[ Epub ahead of print] [60] BHAT M, RABINDRANATH M, CHARA BS, et al. Artificial intelligence, machine learning, and deep learning in liver transplantation[J]. J Hepatol, 2023, 78( 6): 1216- 1233. DOI: 10.1016/j.jhep.2023.01.006. -



 PDF下载 ( 765 KB)
PDF下载 ( 765 KB)

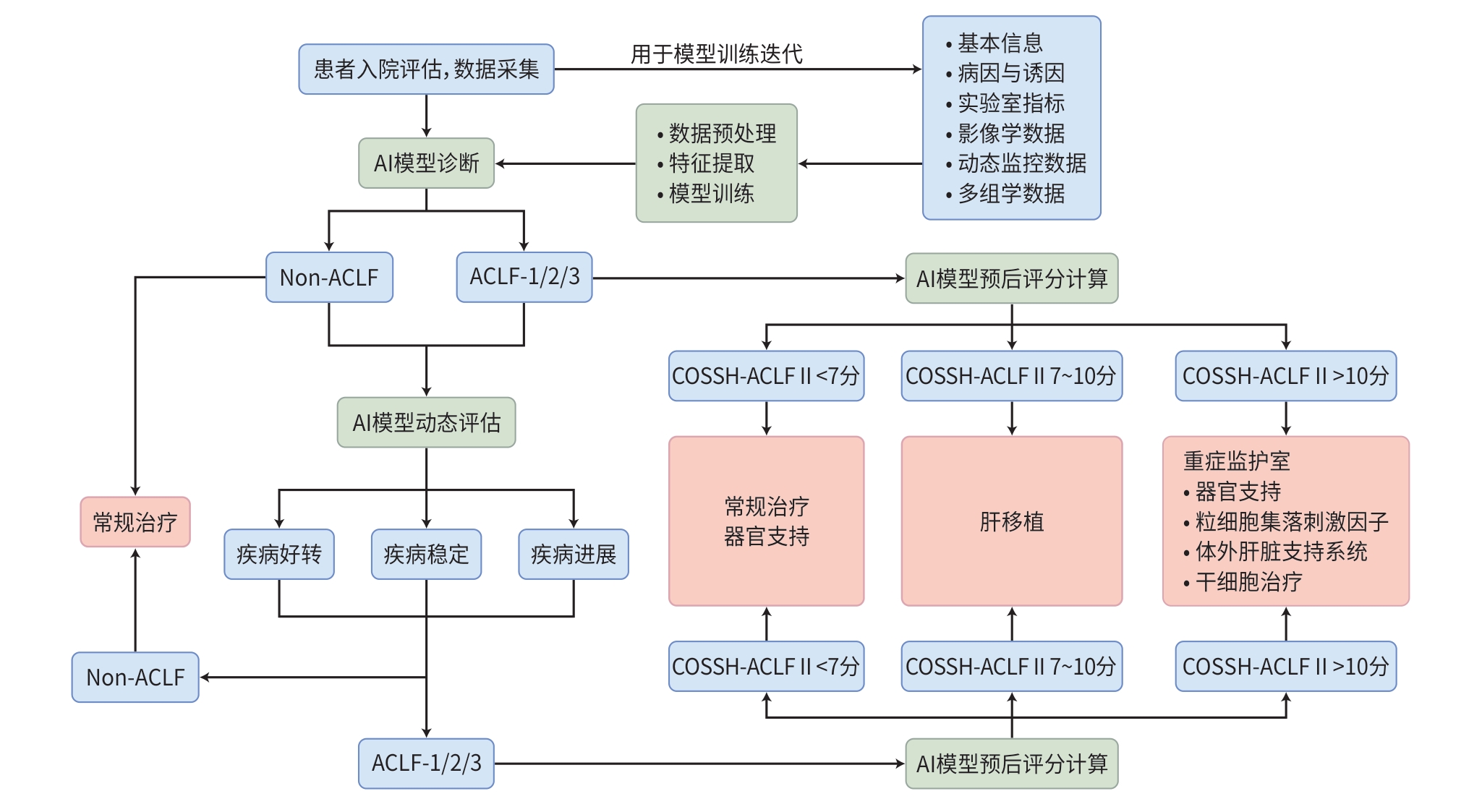
 下载:
下载:


