慢性HBV感染者CD8+T细胞中Mg2+转运蛋白1 mRNA的表达及其与HBV DNA的相关性分析
DOI: 10.12449/JCH251111
The mRNA expression of magnesium transporter 1 in CD8+ T cells and its correlation with HBV DNA in patients with chronic HBV infection
-
摘要:
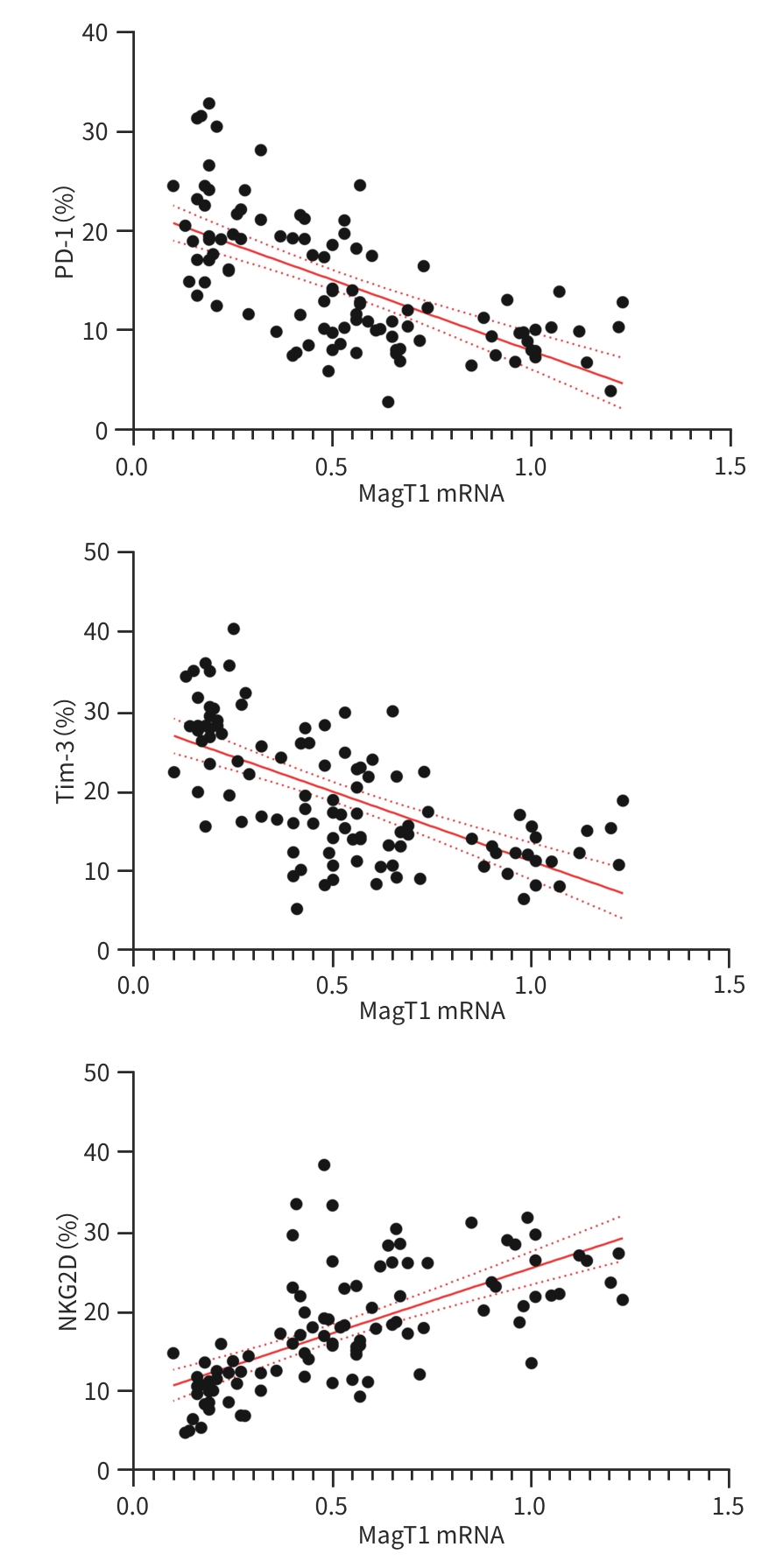
目的 探讨外周血CD8+T细胞中Mg2+转运蛋白1(MagT1)在慢性HBV感染者中的表达变化,并分析血清Mg2+、MagT1 mRNA与HBV DNA载量之间的关系。 方法 选取2022年1月—2023年12月唐山市工人医院收治的HBV感染者102例。采用病例对照设计,将受试对象依据疾病进展阶段划分为3组:慢性乙型肝炎患者40例、代偿期肝硬化患者32例、肝细胞癌患者30例;同时纳入年龄、性别匹配的健康志愿者32例作为正常对照。检测血清Mg2+浓度;使用实时荧光定量PCR检测CD8+T细胞中MagT1 mRNA表达水平及血清HBV DNA载量;采用流式细胞术定量分析CD8+T细胞表面程序性死亡受体1(PD-1)、T细胞免疫球蛋白黏蛋白-3(Tim-3)及自然杀伤细胞活化性受体2D(NKG2D)的表达水平。符合正态分布的计量资料多组间比较采用单因素方差分析,两两比较采用SNK-q检验;计数资料组间比较采用χ2检验;MagT1 mRNA与各指标的相关性采用Pearson线性相关分析。 结果 对照组、慢性乙型肝炎组、代偿期肝硬化组、肝细胞癌组血清Mg2+水平和MagT1 mRNA表达差异均有统计学意义(F值分别为29.014、145.578,P值均<0.001)。Pearson相关性分析显示,血清Mg2+浓度(r=0.335)和MagT 1 mRNA(r=0.394)表达与HBV DNA载量呈正相关(P值均<0.05)。对照组、慢性乙型肝炎组、代偿期肝硬化组、肝细胞癌组PD-1、Tim-3水平依次升高,NKG2D水平依次降低(P值均<0.001)。Pearson相关性分析显示,MagT1 mRNA表达与PD-1(r=-0.643)、Tim-3(r=-0.640)呈负相关,与NKG2D(r=0.655)呈正相关(P值均<0.05)。 结论 HBV感染者外周血CD8+T细胞MagT1 mRNA表达水平下降,且与血清HBV DNA载量呈正相关,MagT1表达降低可能通过阻碍Mg2+内流导致CD8+T细胞功能耗竭,表现为PD-1、Tim-3上调及NKG2D下调。 -
关键词:
- 乙型肝炎病毒 /
- CD8阳性T淋巴细胞 /
- 镁离子转运蛋白1
Abstract:Objective To investigate the change in the expression of magnesium transporter 1 (MagT1) in peripheral blood CD8+ T cells in patients with chronic hepatitis B virus (HBV) infection, as well as the correlation of serum Mg2+ and MagT1 with HBV DNA load. Methods A total of 102 patients with HBV infection who were admitted to Tangshan Workers’ Hospital from January 2022 to December 2023 were enrolled, and a case-control study was conducted. According to the stage of disease progression, the subjects were divided into chronic hepatitis B group with 40 patients, compensated liver cirrhosis group with 32 patients, and hepatocellular carcinoma group with 30 patients, and 32 healthy volunteers matched by age and sex were enrolled as normal control group. The serum concentration of Mg²⁺ was measured; RT-qPCR was used to measure the mRNA expression level of MagT1 in CD8+ T cells and serum HBV DNA load; flow cytometry was used to measure the expression levels of programmed death-1 (PD-1), T-cell immunoglobulin and mucin-domain containing-3 (Tim-3), and natural killer cell group 2D (NKG2D) on the surface of CD8+ T cells. A one-way analysis of variance was used for comparison of normally distributed continuous data between multiple groups, and the SNK-q test was used for comparison between two groups; the chi-square test was used for comparison of categorical data between groups; a Pearson linear correlation analysis was used to investigate the correlation between the mRNA expression level of MagT1 and other related indicators. Results There were significant differences in the serum level of Mg2+ and the mRNA expression level of MagT1 between the normal control group, the chronic hepatitis B group, the compensated liver cirrhosis group, and the hepatocellular carcinoma group (F=29.014 and 145.578, both P<0.001). The Pearson correlation analysis showed that the serum level of Mg2+ and the mRNA expression level of MagT1 were positively correlated with HBV DNA load (r=0.335 and 0.394, both P<0.05). The hepatocellular carcinoma group had the highest levels of PD-1 and Tim-3, followed by the compensated liver cirrhosis group, the chronic hepatitis B group, and the normal control group; the normal control group had the highest level of NKG2D, followed by the chronic hepatitis B group, the compensated liver cirrhosis group, and the hepatocellular carcinoma group (all P<0.001). The Pearson correlation analysis showed that the mRNA expression level of MagT1 was negatively correlated with PD-1 and Tim-3 (r=-0.643 and -0.640, both P<0.05) and was positively correlated with NKG2D (r=0.655, P<0.05). Conclusion There is a reduction in the mRNA expression level of MagT1 in peripheral blood CD8+ T cells in patients with HBV infection, which is positively correlated with serum HBV DNA load. The reduction in the expression of MagT1 may contribute to CD8+ T cell exhaustion by impairing Mg²⁺ influx, manifesting as the upregulation of PD-1 and Tim-3 and the downregulation of NKG2D. -
Key words:
- Hepatitis B Virus /
- CD8-Positive T-Lymphocytes /
- Magnesium Transporter 1
-
表 1 各组受试者一般资料比较
Table 1. Comparison of general information of subjects in each group
组别 例数 性别
(男/女,例)年龄
(岁)BMI
(kg/m2)对照组 32 18/14 40.50±8.10 23.22±2.10 慢性乙型肝炎组 40 20/20 38.52±7.52 22.45±2.55 代偿期肝硬化组 32 15/17 41.21±5.55 22.20±2.50 肝细胞癌组 30 15/15 43.20±8.33 21.89±2.55 统计值 χ2=0.801 F=2.328 F=1.704 P值 0.301 0.078 0.169 表 2 各组血清Mg2+浓度、MagT1 mRNA和HBV DNA水平比较
Table 2. Comparison of serum Mg2+ concentration, MagT1 mRNA and HBV DNA levels in each group
组别 例数 Mg2+(mmol/L) MagT1 mRNA HBV DNA(log10 IU/mL) 对照组 32 0.96±0.10 1.25±0.30 慢性乙型肝炎组 40 0.90±0.061) 0.80±0.251) 6.89±2.01 代偿期肝硬化组 32 0.86±0.061)2) 0.50±0.101)2) 5.33±1.502) 肝细胞癌组 30 0.80±0.051)2)3) 0.20±0.051)2)3) 4.33±1.202)3) F值 29.014 145.578 21.533 P值 <0.001 <0.001 <0.001 注:与对照组比较,1)P<0.05;与慢性乙型肝炎组比较,2)P<0.05;与代偿期肝硬化组比较,3)P<0.05。
表 3 各组CD8+T细胞表面分子表达水平比较
Table 3. Comparison of surface molecular expression levels of CD8+T cells in each group
组别 例数 PD-1(%) Tim-3(%) NKG2D(%) 对照组 32 5.30±1.81 8.20±2.10 30.00±4.05 慢性乙型肝炎组 40 8.96±2.331) 12.34±3.401) 25.01±5.501) 代偿期肝硬化组 32 15.55±4.501)2) 20.32±5.501)2) 16.32±3.451)2) 肝细胞癌组 30 21.50±5.551)2)3) 28.34±5.851)2)3) 10.22±2.801)2)3) F值 120.815 130.010 140.699 P值 <0.001 <0.001 <0.001 注:与对照组比较,1)P<0.05;与慢性乙型肝炎组比较,2)P<0.05;与代偿期肝硬化组比较,3)P<0.05。
-
[1] JIA HY, LIU KZ. Antiviral therapy for patients in the immune-tolerant phase of chronic HBV infection is beneficial for preventing liver cirrhosis and hepatocellular carcinoma[J]. J Clin Hepatol, 2021, 37( 5): 1024- 1025. DOI: 10.3969/j.issn.1001-5256.2021.05.007.贾红宇, 刘克洲. 慢性HBV感染免疫耐受期抗病毒治疗有益于预防肝硬化和肝癌的发生[J]. 临床肝胆病杂志, 2021, 37( 5): 1024- 1025. DOI: 10.3969/j.issn.1001-5256.2021.05.007. [2] HSU YC, HUANG DQ, NGUYEN MH. Global burden of hepatitis B virus: Current status, missed opportunities and a call for action[J]. Nat Rev Gastroenterol Hepatol, 2023, 20( 8): 524- 537. DOI: 10.1038/s41575-023-00760-9. [3] CUI FQ, BLACH S, MANZENGO MINGIEDI C, et al. Global reporting of progress towards elimination of hepatitis B and hepatitis C[J]. Lancet Gastroenterol Hepatol, 2023, 8( 4): 332- 342. DOI: 10.1016/S2468-1253(22)00386-7. [4] ZHANG L, FAN ZF, LIU DW, et al. Trend analysis on the disease burden related to cirrhosis and other chronic liver diseases caused by hepatitis B, in China, from 1990 to 2016[J]. Chin J Epidemiol, 2020, 41( 2): 173- 177. DOI: 10.3760/cma.j.issn.0254-6450.2020.02.007.张露, 范志芳, 刘殿武, 等. 1990—2016年中国乙型肝炎引起的肝硬化及其他慢性肝病疾病负担变化趋势分析[J]. 中华流行病学杂志, 2020, 41( 2): 173- 177. DOI: 10.3760/cma.j.issn.0254-6450.2020.02.007. [5] SPONDER G, SVIDOVA S, SCHINDL R, et al. Lpe10p modulates the activity of the Mrs2p-based yeast mitochondrial Mg2+ channel[J]. FEBS J, 2010, 277( 17): 3514- 3525. DOI: 10.1111/j.1742-4658.2010.07761.x. [6] GOYTAIN A, HINES RM, QUAMME GA. Huntingtin-interacting proteins, HIP14 and HIP14L, mediate dual functions, palmitoyl acyltransferase and Mg2+ transport[J]. J Biol Chem, 2008, 283( 48): 33365- 33374. DOI: 10.1074/jbc.M801469200. [7] MO Q, YUAN Z, NING L, et al. miR-199a-5p targeted regulation of MAGT1 expression in the functional depletion of CD8+T cells in HBV infection[J]. Magnes Res, 2020, 33( 3): 58- 67. DOI: 10.1684/mrh.2020.0469. [8] YUAN ZL, HOU W. Study on the molecular mechanism of MagT1 regulating activated T cell in chronic hepatitis virus infection[J]. Mil Med J South China, 2019, 33( 4): 238- 242. DOI: 10.13730/j.issn.1009-2595.2019.04.004.袁紫林, 侯炜. 慢性HBV感染中MagT1调节活化T细胞功能的分子机制研究[J]. 华南国防医学杂志, 2019, 33( 4): 238- 242. DOI: 10.13730/j.issn.1009-2595.2019.04.004. [9] Expert Group for Project of Hepatocellular Carcinoma Screening and Surveillance,Chinese Foundation for Hepatitis Prevention and Control. Screening and surveillance for hepatocellular carcinoma in patients with chronic hepatitis B virus infection[J]. J Pract Hepatol, 2021, 24( 6): 776- 785. DOI: 10.3969/j.issn.1672-5069.2021.06.004.中国肝炎防治基金会肝细胞癌筛查和监测项目专家组. 慢性乙型肝炎病毒感染者肝细胞癌筛查和监测[J]. 实用肝脏病杂志, 2021, 24( 6): 776- 785. DOI: 10.3969/j.issn.1672-5069.2021.06.004. [10] XIE YD, FENG B, RAO HY. Interpretation of guidelines for the prevention and treatment of chronic hepatitis B(2022 edition)[J]. J Clin Hepatol, 2023, 39( 7): 1553- 1559. DOI: 10.3969/j.issn.1001-5256.2023.07.007.谢艳迪, 封波, 饶慧瑛.《慢性乙型肝炎防治指南(2022年版)》解读[J]. 临床肝胆病杂志, 2023, 39( 7): 1553- 1559. DOI: 10.3969/j.issn.1001-5256.2023.07.007. [11] Chinese Society of Hepatology, Chinese Medical Association. Chinese guidelines on the management of liver cirrhosis[J]. J Clin Hepatol, 2019, 35( 11): 2408- 2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006.中华医学会肝病学分会. 肝硬化诊治指南[J]. 临床肝胆病杂志, 2019, 35( 11): 2408- 2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006. [12] General Office of National Health Commission. Standard for diagnosis and treatment of primary liver cancer(2022 edition)[J]. J Clin Hepatol, 2022, 38( 2): 288- 303. DOI: 10.3969/j.issn.1001-5256.2022.02.009.国家卫生健康委办公厅. 原发性肝癌诊疗指南(2022年版)[J]. 临床肝胆病杂志, 2022, 38( 2): 288- 303. DOI: 10.3969/j.issn.1001-5256.2022.02.009. [13] LIN XE, WU JH, WANG N, et al. The effect of Mg2+ transporter 1 expression level on CD8+T cell exhaustion in patients with chronic hepatitis B virus infection[J]. J Prac Med, 2019, 35( 13): 2070- 2073, 2077. DOI: 10.3969/j.issn.1006-5725.2019.13.009.林晓娥, 吴景华, 王宁, 等. 慢性乙型肝炎病毒感染者Mg2+转运蛋白1表达水平对CD8+T细胞耗竭的影响[J]. 实用医学杂志, 2019, 35( 13): 2070- 2073, 2077. DOI: 10.3969/j.issn.1006-5725.2019.13.009. [14] TSAI KN, KUO CF, OU JJ. Mechanisms of hepatitis B virus persistence[J]. Trends Microbiol, 2018, 26( 1): 33- 42. DOI: 10.1016/j.tim.2017.07.006. [15] LIN HY, WANG LH, LIN HX, et al. Relationship between polymorphisms of p53 pathway genes and postoperative virus activation in patients with HBV infection related hepatocellular carcinoma[J]. Chin J Nosocomiology, 2022, 32( 10): 1473- 1477. DOI: 10.11816/cn.ni.2022-211372.林海燕, 王丽华, 林红霞, 等. p53通路基因多态性与HBV感染相关肝细胞癌患者术后病毒激活的关联[J]. 中华医院感染学杂志, 2022, 32( 10): 1473- 1477. DOI: 10.11816/cn.ni.2022-211372. [16] WU Y, LIU Z, ZHANG R, et al. Relationship of refeeding syndrome with cellular immune function and nosocomial death in severe emergency patients[J]. Zhejiang Med J, 2022, 44( 22): 2394- 2399, 2423. DOI: 10.12056/j.issn.1006-2785.2022.44.22.2022-531.邬媛, 刘仲, 张茹, 等. 再喂养综合征与急诊重症患者细胞免疫功能以及院内死亡的关系分析[J]. 浙江医学, 2022, 44( 22): 2394- 2399, 2423. DOI: 10.12056/j.issn.1006-2785.2022.44.22.2022-531. [17] FRITZEN R, DAVIES A, VEENHUIZEN M, et al. Magnesium deficiency and cardiometabolic disease[J]. Nutrients, 2023, 15( 10): 2355. DOI: 10.3390/nu15102355. [18] MATSUDA-LENNIKOV M, BIANCALANA M, ZOU J, et al. Magnesium transporter 1(MAGT1) deficiency causes selective defects in N-linked glycosylation and expression of immune-response genes[J]. J Biol Chem, 2019, 294( 37): 13638- 13656. DOI: 10.1074/jbc.RA119.008903. [19] GOLLOSHI K, MITCHELL W, KUMAR D, et al. HLH and recurrent EBV lymphoma as the presenting manifestation of MAGT1 deficiency: A systematic review of the expanding disease spectrum[J]. J Clin Immunol, 2024, 44( 7): 153. DOI: 10.1007/s10875-024-01749-y. [20] WANG JC, ZHOU M, ZHOU JF, et al. Monozygotic twins with MAGT1 deficiency and Epstein-Barr virus-positive classic Hodgkin lymphoma receiving anti-CD30 CAR T-cell immunotherapy: A case report[J]. J Clin Immunol, 2024, 44( 4): 91. DOI: 10.1007/s10875-024-01690-0. [21] KAUSKOT A, MALLEBRANCHE C, BRUNEEL A, et al. MAGT1 deficiency in XMEN disease is associated with severe platelet dysfunction and impaired platelet glycoprotein N-glycosylation[J]. J Thromb Haemost, 2023, 21( 11): 3268- 3278. DOI: 10.1016/j.jtha.2023.05.007. [22] LI FY, LENARDO MJ, CHAIGNE-DELALANDE B. Loss of MAGT1 abrogates the Mg2+ flux required for T cell signaling and leads to a novel human primary immunodeficiency[J]. Magnes Res, 2011, 24( 3): 109- 114. DOI: 10.1684/mrh.2011.0286. [23] XU DQ, FU HH, OBAR JJ, et al. A potential new pathway for PD-L1 costimulation of the CD8-T cell response to Listeria monocytogenes infection[J]. PLoS One, 2013, 8( 2): e56539. DOI: 10.1371/journal.pone.0056539. [24] DUCKER CE, STETTLER EM, FRENCH KJ, et al. Huntingtin interacting protein 14 is an oncogenic human protein: Palmitoyl acyltransferase[J]. Oncogene, 2004, 23( 57): 9230- 9237. DOI: 10.1038/sj.onc.1208171. [25] SCHINDL R, WEGHUBER J, ROMANIN C, et al. Mrs2p forms a high conductance Mg2+ selective channel in mitochondria[J]. Biophys J, 2007, 93( 11): 3872- 3883. DOI: 10.1529/biophysj.107.112318. [26] HUANG QZ, WU X, WANG ZM, et al. The primordial differentiation of tumor-specific memory CD8+ T cells as bona fide responders to PD-1/PD-L1 blockade in draining lymph nodes[J]. Cell, 2022, 185( 22): 4049- 4066. e 25. DOI: 10.1016/j.cell.2022.09.020. [27] DIXON KO, LAHORE GF, KUCHROO VK. Beyond T cell exhaustion: TIM-3 regulation of myeloid cells[J]. Sci Immunol, 2024, 9( 93): eadf2223. DOI: 10.1126/sciimmunol.adf2223. [28] MOJIC M, SHITAOKA K, OHSHIMA C, et al. NKG2D defines tumor-reacting effector CD8+ T cells within tumor microenvironment[J]. Cancer Sci, 2021, 112( 9): 3484- 3490. DOI: 10.1111/cas.15050. [29] KANELLOPOULOU C, GEORGE AB, MASUTANI E, et al. Mg2+ regulation of kinase signaling and immune function[J]. J Exp Med, 2019, 216( 8): 1828- 1842. DOI: 10.1084/jem.20181970. [30] LÖTSCHER J, MARTÍ I LÍNDEZ AA, KIRCHHAMMER N, et al. Magnesium sensing via LFA-1 regulates CD8+ T cell effector function[J]. Cell, 2022, 185( 4): 585- 602. e 29. DOI: 10.1016/j.cell.2021.12.039. [31] TOMITA A, ZHANG MF, JIN F, et al. ATP-dependent modulation of MgtE in Mg2+ homeostasis[J]. Nat Commun, 2017, 8: 148. DOI: 10.1038/s41467-017-00082-w. -



 PDF下载 ( 1488 KB)
PDF下载 ( 1488 KB)


 下载:
下载: