达格列净对代谢相关脂肪性肝病小鼠模型肠道菌群的影响及其机制分析
DOI: 10.12449/JCH251116
Effect and mechanism of dapagliflozin on gut microbiota in a mouse model of metabolic associated fatty liver disease
-
摘要:
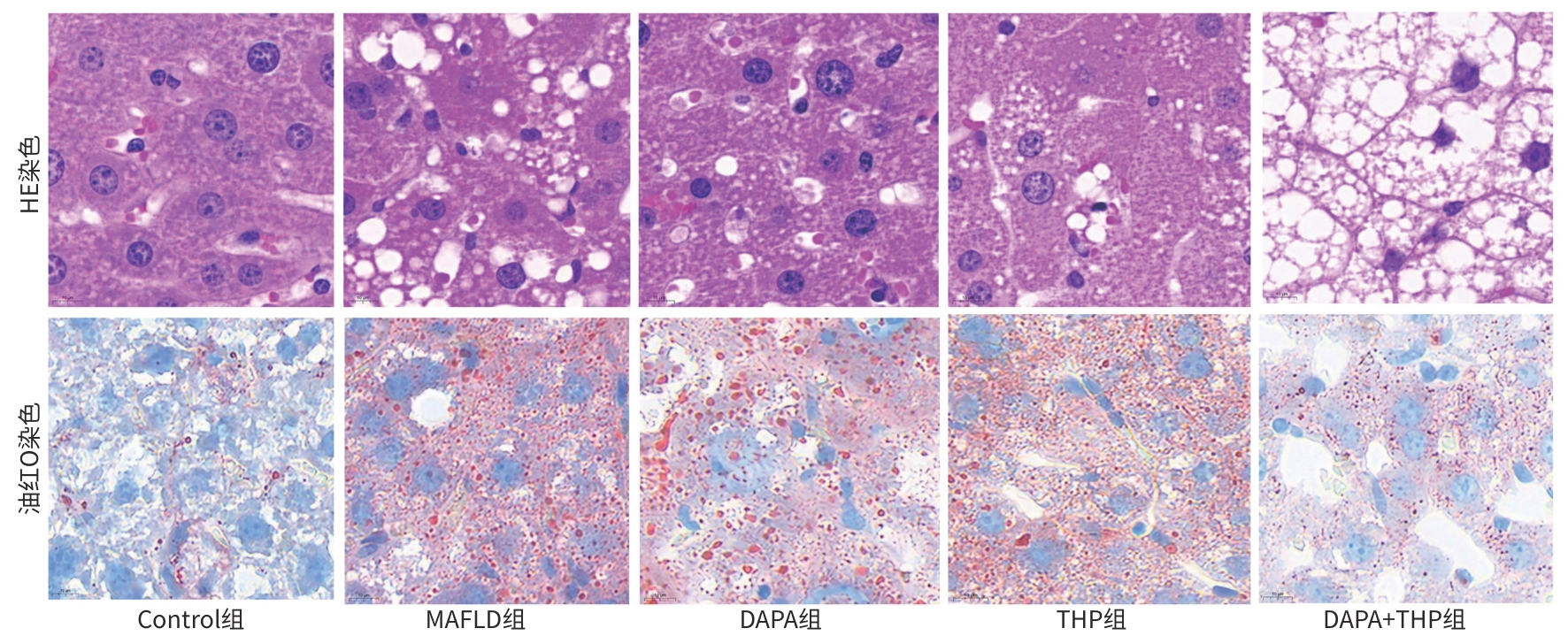
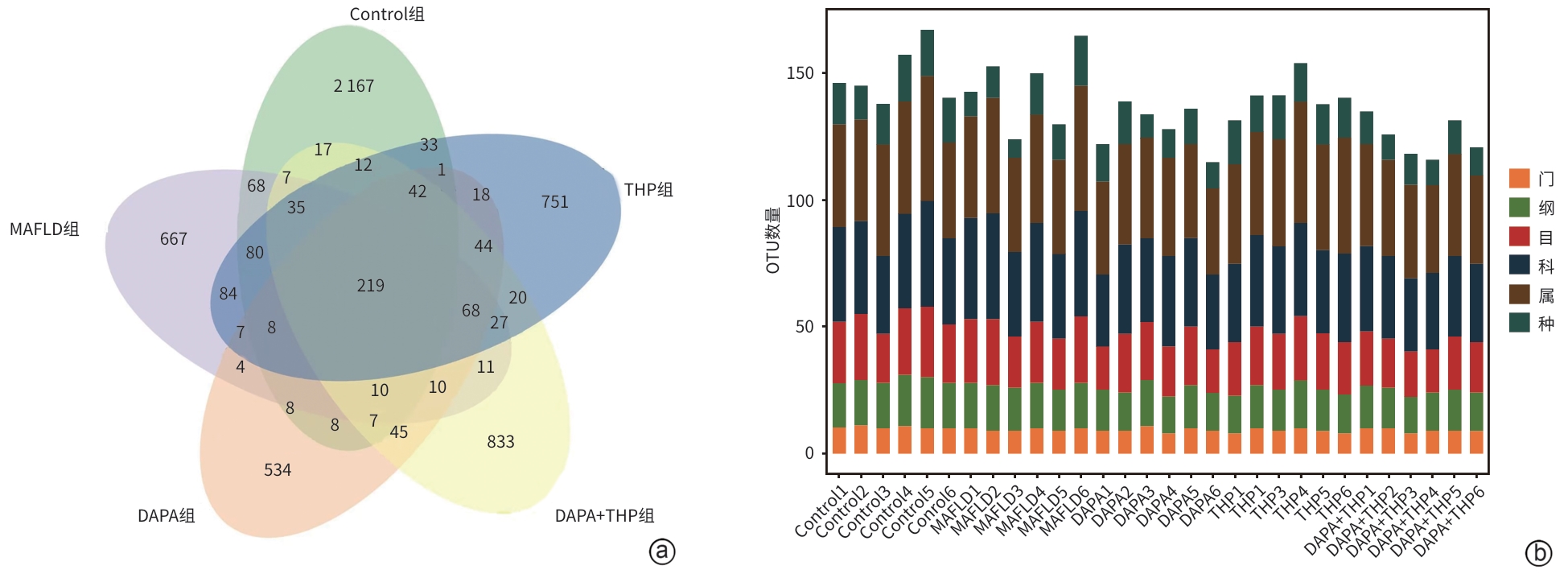
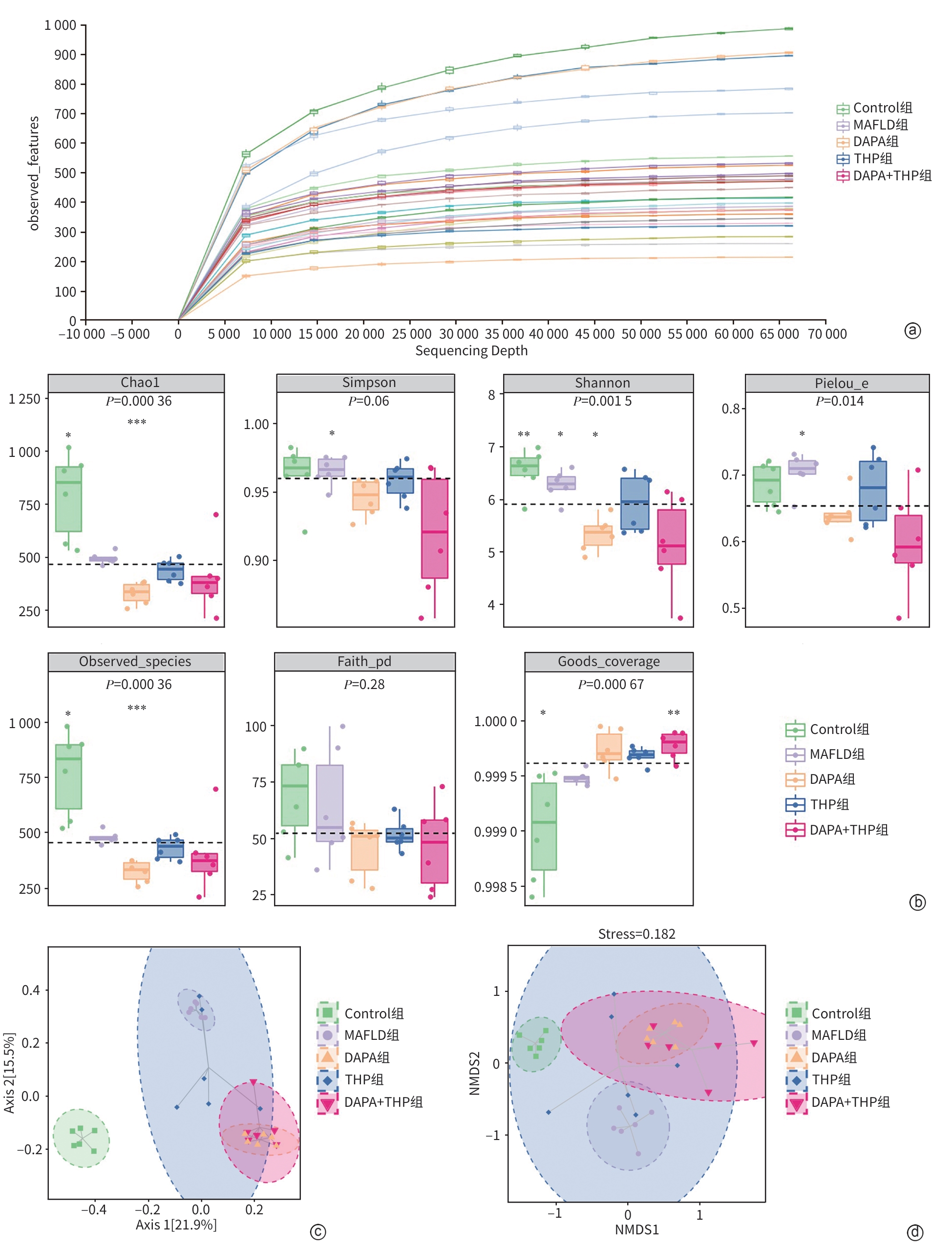
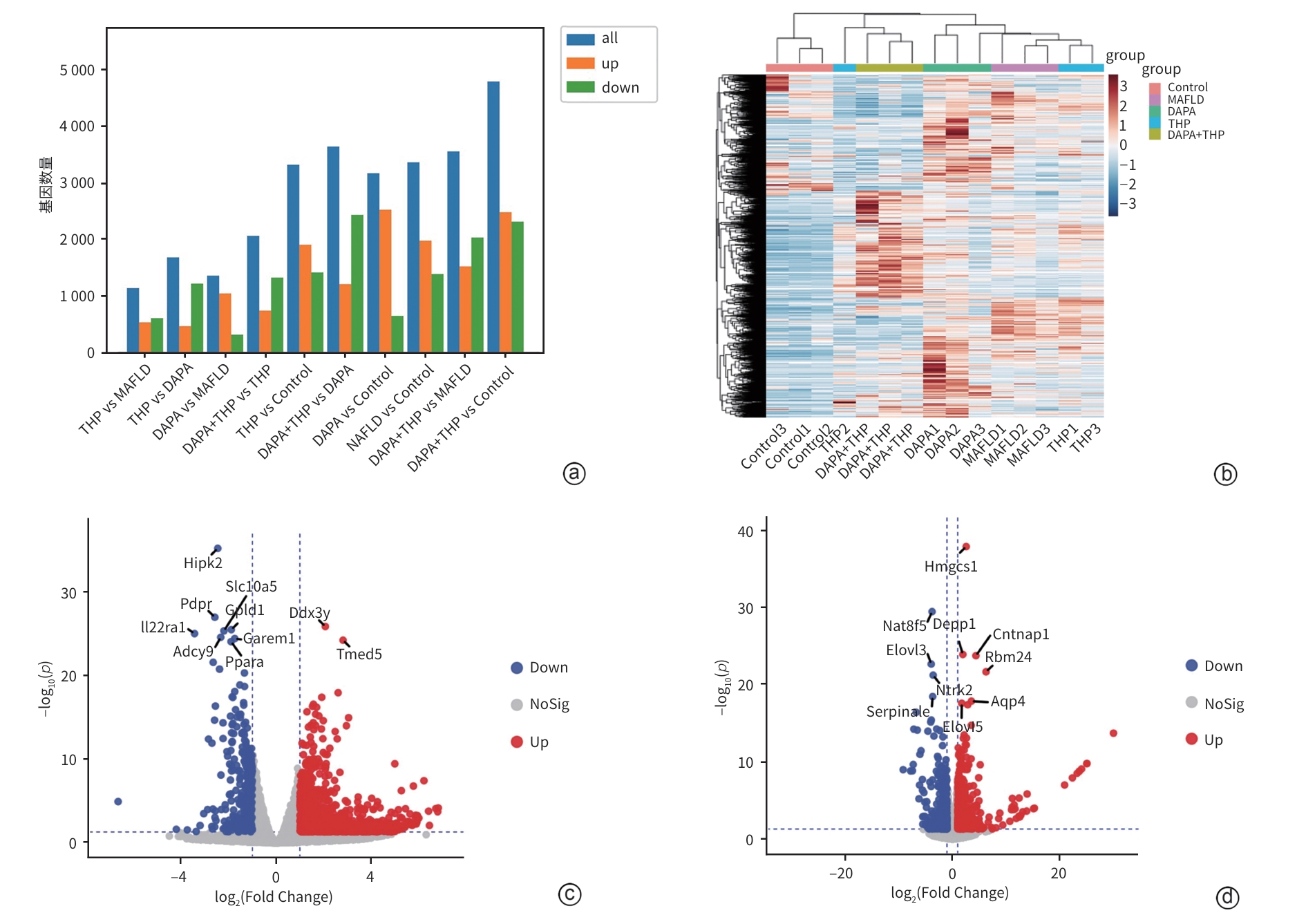
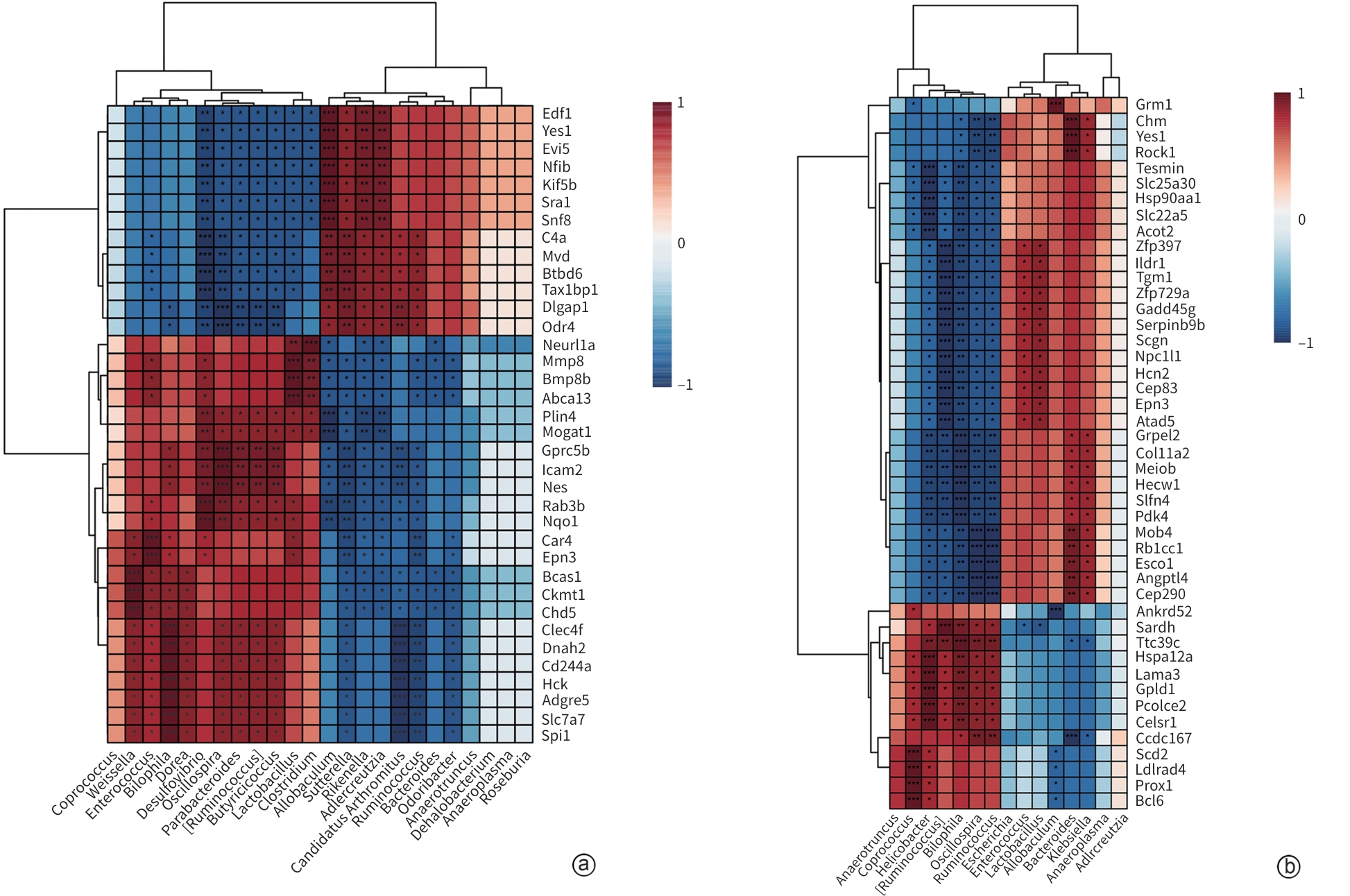
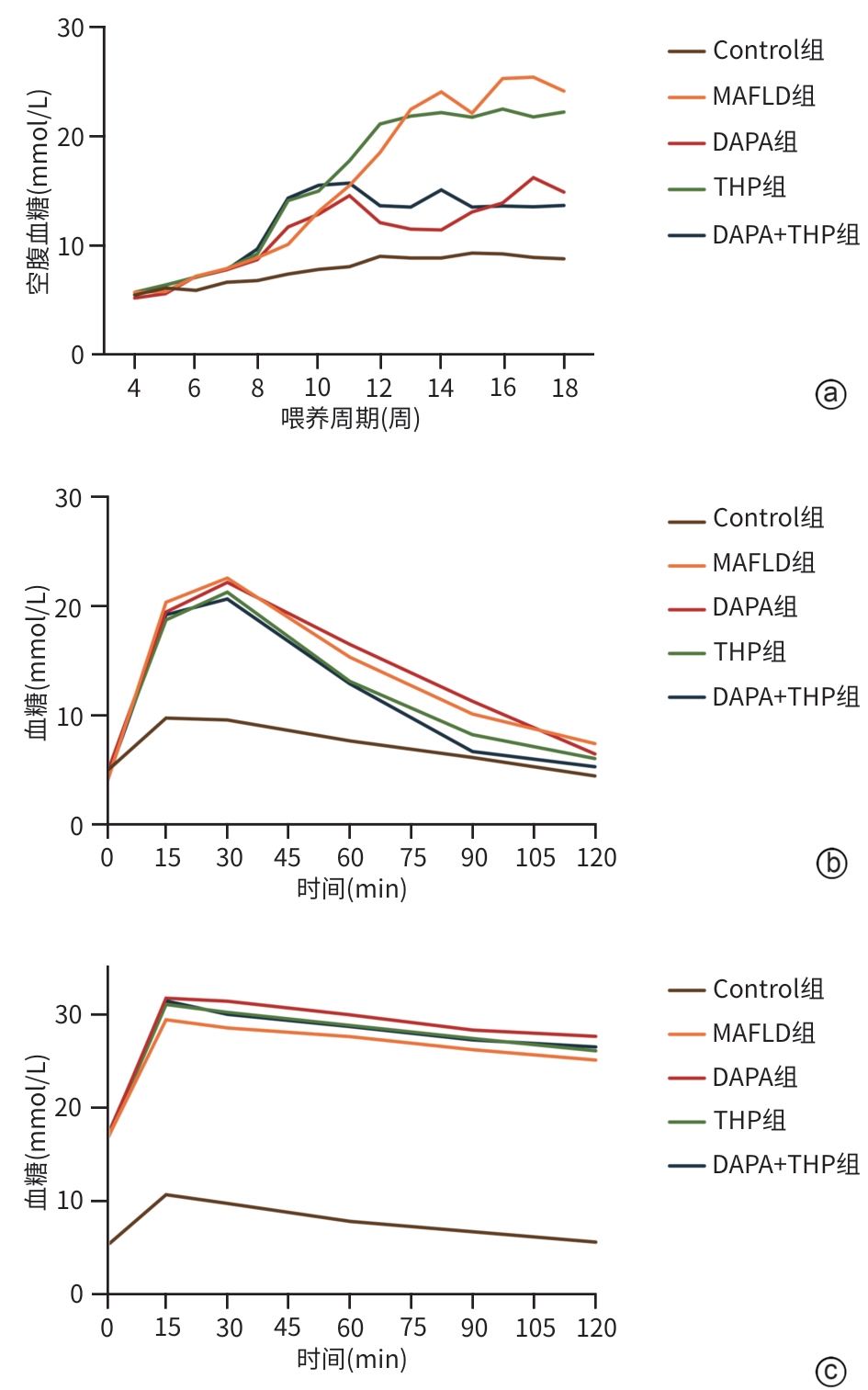
目的 探讨达格列净对代谢相关脂肪性肝病(MAFLD)小鼠肝脂代谢及肠道微生态的影响,并阐明其潜在作用机制。 方法 50只C57雄性小鼠随机分为正常组(Control组)、2型糖尿病合并代谢相关脂肪性肝病组(MAFLD组)、达格列净组(DAPA组)、米屈肼组(THP组)、达格列净联合米屈肼组(DAPA+THP组),每组各10只。采用高脂饮食联合链脲佐菌素诱导MAFLD小鼠模型。通过组织病理学、血糖血脂等生化指标评估疗效,并利用转录组学和宏基因组学分析差异基因及菌群变化。正态分布的计量资料多组间比较采用单因素方差分析,两两比较采用LSD检验;非正态分布的计量资料多组间比较采用Kruskal-Wallis H检验,两两比较采用Nemenyi检验。 结果 组织病理学结果显示,MAFLD组小鼠表现为过量的脂质沉积,肝细胞出现脂肪样变;与MAFLD组相比,DAPA组肝细胞脂肪样变显著改善,THP组、DAPA+THP组改善效果无DAPA组明显。与Control组相比,MAFLD组空腹血糖显著升高(P<0.05),血清ALT、AST、MDA、TC、TG、LDL-C均显著升高(P值均<0.05),HDL-C明显降低(P<0.05)。与MAFLD组相比,DAPA组、THP组和DAPA+THP组血清ALT、AST显著降低(P值均<0.05)。16S rRNA测序显示,MAFLD组较Control组,小鼠肠道菌群发生显著改变,MAFLD组的Firmicutes、Lactobacillaceae明显增加,Bacteroidetes、S24-7和Erysipelotrichaceae明显减少。DAPA组、THP组和DAPA+THP组可调节上述菌群趋向正常水平。肝脏转录组学分析主要富集的代谢通路包括类固醇激素生物合成、胆汁分泌、炎性介质调节TRP(瞬时受体电位通道)、脂肪酸延伸、脂质生物代谢过程等,关联的基因主要涉及脂质代谢关键靶点Acot2、Angptl4、Scd2和Npc1l1。 结论 DAPA可能通过类固醇激素生物合成、胆汁分泌、炎性介质调节TRP、脂肪酸延伸通路改善MAFLD,调节肠道菌群稳态。 Abstract:Objective To investigate the effect of dapagliflozin on liver lipid metabolism and gut microecology in mice with metabolic associated fatty liver disease (MAFLD), and to clarify its potential mechanism. Methods A total of 50 male C57 mice were randomly divided into Control group, type 2 diabetes+MAFLD group (MAFLD group), dapagliflozin group (DAPA group), meldonium group (THP group), and dapagliflozin+meldonium group (DAPA+THP group), with 10 mice in each group. High-fat diet combined with streptozotocin was used to establish a mouse model of MAFLD. Treatment outcomes were assessed based on histopathology and biochemical parameters such as blood glucose and blood lipid levels, and the transcriptomic and metagenomic analyses were used to identify differentially expressed genes and the changes in gut microbiota. A one-way analysis of variance was used for comparison of normally distributed continuous data between multiple groups, and the least significant difference t-test was used for comparison between two groups; the Kruskal-Wallis H test was used for comparison of non-normally distributed continuous data between multiple groups, and the Nemenyi test was used for comparison between two groups. Results Histopathological examination showed that the mice in the MAFLD group had excessive lipid deposition and hepatocyte steatosis; compared with the MAFLD group, the DAPA group had a significant improvement in hepatocyte steatosis, while the THP group and the DAPA+THP group had a less significant improvement compared with the DAPA group. Compared with the Control group, the MAFLD group had a significant increase in fasting blood glucose (P<0.05), significant increases in the serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), malondialdehyde, total cholesterol, triglyceride, and low-density lipoprotein cholesterol (P<0.05), and a significant reduction in high-density lipoprotein cholesterol (P<0.05). Compared with the MAFLD group, the DAPA group, the THP group, and the DAPA+THP group had significant reductions in the serum levels of ALT and AST (P<0.05). The results of 16S rRNA sequencing showed that compared with the Control group, the MAFLD group had significant changes in gut microbiota, with an increase in Firmicutes and a reduction in Bacteroidetes, as well as reductions in S24-7 and Erysipelotrichaceae and an increase in Lactobacillaceae. The levels of the above flora were upregulated to normal levels in the DAPA group, the THP group, and the DAPA+THP group. The liver transcriptomic analysis showed that the enriched metabolic pathways included steroid hormone biosynthesis, bile secretion, inflammatory mediator regulation of TRP, fatty acid elongation, and lipid biodegradation processes, and the related genes mainly involved the key targets of lipid metabolism such as Acot2, Angptl4, Scd2, and Npc1l1. Conclusion Dapagliflozin can alleviate MAFLD through the pathways such as steroid hormone biosynthesis, bile secretion, inflammatory mediator regulation of TRP, and fatty acid elongation, as well as by regulating gut microbiota homeostasis. -
表 1 小鼠生化指标情况
Table 1. Biochemical indicators of mice
指标 Control组 MAFLD组 DAPA组 THP组 DAPA+THP组 H值 P值 ALT(U/L) 85.34
(84.43~85.48)87.33
(86.66~87.81)1)85.04
(84.81~85.40)2)85.01
(84.02~85.72)2)82.56
(81.60~83.59)1)2)24.15 <0.001 AST(U/L) 16.02
(15.90~16.15)17.21
(16.97~17.35)1)16.21
(15.64~16.43)2)16.09
(15.84~16.43)1)2)15.93
(15.66~16.23)2)20.81 0.001 GSH(μmol/L) 65.63
(48.96~112.15)10.42
(8.33~50.69)54.34
(53.91~75.17)85.42
(59.20~107.12)53.13
(50.95~55.99)9.37 0.053 MDA(nmol/mL) 29.39
(18.91~44.65)261.28
(244.65~325.51)1)41.12
(30.52~53.25)2)318.91
(299.37~353.25)1)26.99
(15.49~30.64)23.53 <0.001 SOD(U/mL) 19.88
(19.13~21.85)18.62
(14.31~21.42)22.06
(20.65~23.82)2)19.48
(18.91~20.38)25.14
(23.73~26.42)1)2)19.35 0.001 TC(mmol/L) 6.89
(6.63~7.19)8.81
(8.28~10.06)1)7.27
(6.59~7.63)2)9.82
(9.50~10.19)2)7.05
(6.70~8.46)2)20.37 0.001 TG(mmol/L) 0.96
(0.89~0.97)1.56
(1.49~1.63)1)1.07
(0.90~1.14)2)1.04
(0.75~1.18)1)0.77
(0.73~0.78)2)18.16 0.001 HDL-C(mmol/L) 3.37
(3.14~3.44)2.38
(2.21~2.59) 1)3.00
(2.81~3.03)2.84
(2.53~3.49)2.52
(2.45~3.09)14.43 0.006 LDL-C(mmol/L) 4.62
(4.47~4.99)7.59
(7.11~8.27)1)4.89
(4.64~5.14)4.62
(4.53~4.72)6.19
(6.00~6.45)22.79 0.000 1 注:与Control组比较,1)P<0.05;与MAFLD组比较,2)P<0.05。
-
[1] YOUNOSSI ZM, LOOMBA R, RINELLA ME, et al. Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis[J]. Hepatology, 2018, 68( 1): 361- 371. DOI: 10.1002/hep.29724. [2] YOUNOSSI Z, TACKE F, ARRESE M, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis[J]. Hepatology, 2019, 69( 6): 2672- 2682. DOI: 10.1002/hep.30251. [3] YABIKU K, MUTOH A, MIYAGI K, et al. Effects of oral antidiabetic drugs on changes in the liver-to-spleen ratio on computed tomography and inflammatory biomarkers in patients with type 2 diabetes and nonalcoholic fatty liver disease[J]. Clin Ther, 2017, 39( 3): 558- 566. DOI: 10.1016/j.clinthera.2017.01.015. [4] DONG YJ, LV QG, LI SY, et al. Efficacy and safety of glucagon-like peptide-1 receptor agonists in non-alcoholic fatty liver disease: A systematic review and meta-analysis[J]. Clin Res Hepatol Gastroenterol, 2017, 41( 3): 284- 295. DOI: 10.1016/j.clinre.2016.11.009. [5] NEWSOME PN, BUCHHOLTZ K, CUSI K, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis[J]. N Engl J Med, 2021, 384( 12): 1113- 1124. DOI: 10.1056/NEJMoa2028395. [6] RAJ H, DURGIA H, PALUI R, et al. SGLT-2 inhibitors in non-alcoholic fatty liver disease patients with type 2 diabetes mellitus: A systematic review[J]. World J Diabetes, 2019, 10( 2): 114- 132. DOI: 10.4239/wjd.v10.i2.114. [7] FERRANNINI E, RAMOS SJ, SALSALI A, et al. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: A randomized, double-blind, placebo-controlled, phase 3 trial[J]. Diabetes Care, 2010, 33( 10): 2217- 2224. DOI: 10.2337/dc10-0612. [8] DHILLON S. Dapagliflozin: A review in type 2 diabetes[J]. Drugs, 2019, 79( 10): 1135- 1146. DOI: 10.1007/s40265-019-01148-3. [9] BAYS HE, SARTIPY P, XU J, et al. Dapagliflozin in patients with type II diabetes mellitus, with and without elevated triglyceride and reduced high-density lipoprotein cholesterol levels[J]. J Clin Lipidol, 2017, 11( 2): 450- 458. e 1. DOI: 10.1016/j.jacl.2017.01.018. [10] SUIJK DLS, VAN BAAR MJB, VAN BOMMEL EJM, et al. SGLT2 inhibition and uric acid excretion in patients with type 2 diabetes and normal kidney function[J]. Clin J Am Soc Nephrol, 2022, 17( 5): 663- 671. DOI: 10.2215/CJN.11480821. [11] JAIKUMKAO K, PONGCHAIDECHA A, CHUEAKULA N, et al. Dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, slows the progression of renal complications through the suppression of renal inflammation, endoplasmic reticulum stress and apoptosis in prediabetic rats[J]. Diabetes Obes Metab, 2018, 20( 11): 2617- 2626. DOI: 10.1111/dom.13441. [12] WIVIOTT SD, RAZ I, BONACA MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes[J]. N Engl J Med, 2019, 380( 4): 347- 357. DOI: 10.1056/NEJMoa1812389. [13] LIEPINSH E, KUKA J, SVALBE B, et al. Effects of long-term mildronate treatment on cardiac and liver functions in rats[J]. Basic Clin Pharmacol Toxicol, 2009, 105( 6): 387- 394. DOI: 10.1111/j.1742-7843.2009.00461.x. [14] HMELNICKIS J, PUGOVICS O, KAZOKA H, et al. Application of hydrophilic interaction chromatography for simultaneous separation of six impurities of mildronate substance[J]. J Pharm Biomed Anal, 2008, 48( 3): 649- 656. DOI: 10.1016/j.jpba.2008.06.011. [15] DE NICOLA L, GABBAI FB, GAROFALO C, et al. Nephroprotection by SGLT2 inhibition: Back to the future?[J]. J Clin Med, 2020, 9( 7): 2243. DOI: 10.3390/jcm9072243. [16] XU L, NAGATA N, NAGASHIMADA M, et al. SGLT2 inhibition by empagliflozin promotes fat utilization and browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in diet-induced obese mice[J]. EBioMedicine, 2017, 20: 137- 149. DOI: 10.1016/j.ebiom.2017.05.028. [17] KUSMINSKI CM, MCTERNAN PG, SCHRAW T, et al. Adiponectin complexes in human cerebrospinal fluid: Distinct complex distribution from serum[J]. Diabetologia, 2007, 50( 3): 634- 642. DOI: 10.1007/s00125-006-0577-9. [18] TAHARA A, TAKASU T. Therapeutic effects of SGLT2 inhibitor ipragliflozin and metformin on NASH in type 2 diabetic mice[J]. Endocr Res, 2020, 45( 2): 147- 161. DOI: 10.1080/07435800.2020.1713802. [19] WANG JX, RAHIMNEJAD S, ZHANG YY, et al. Mildronate triggers growth suppression and lipid accumulation in largemouth bass(Micropterus salmoides) through disturbing lipid metabolism[J]. Fish Physiol Biochem, 2022, 48( 1): 145- 159. DOI: 10.1007/s10695-021-01040-6. [20] BOURSIER J, MUELLER O, BARRET M, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota[J]. Hepatology, 2016, 63( 3): 764- 775. DOI: 10.1002/hep.28356. [21] LIU RX, HONG J, XU XQ, et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention[J]. Nat Med, 2017, 23( 7): 859- 868. DOI: 10.1038/nm.4358. [22] HSU CL, SCHNABL B. The gut-liver axis and gut microbiota in health and liver disease[J]. Nat Rev Microbiol, 2023, 21( 11): 719- 733. DOI: 10.1038/s41579-023-00904-3. [23] CANFORA EE, JOCKEN JW, BLAAK EE. Short-chain fatty acids in control of body weight and insulin sensitivity[J]. Nat Rev Endocrinol, 2015, 11( 10): 577- 591. DOI: 10.1038/nrendo.2015.128. [24] SYLVERS-DAVIE KL, DAVIES BSJ. Regulation of lipoprotein metabolism by ANGPTL3, ANGPTL4, and ANGPTL8[J]. Am J Physiol Endocrinol Metab, 2021, 321( 4): E493- E508. DOI: 10.1152/ajpendo.00195.2021. [25] WANG BH, JIANG XY, CAO M, et al. Altered fecal microbiota correlates with liver biochemistry in nonobese patients with non-alcoholic fatty liver disease[J]. Sci Rep, 2016, 6: 32002. DOI: 10.1038/srep32002. [26] HUNT MC, TILLANDER V, ALEXSON SEH. Regulation of peroxisomal lipid metabolism: The role of acyl-CoA and coenzyme A metabolizing enzymes[J]. Biochimie, 2014, 98: 45- 55. DOI: 10.1016/j.biochi.2013.12.018. [27] MA S, SHI S, XU BH, et al. Host serine protease ACOT2 assists DENV proliferation by hydrolyzing viral polyproteins[J]. mSystems, 2024, 9( 1): e00973-23. DOI: 10.1128/msystems.00973-23. [28] ALTMANN SW, DAVIS HR Jr, ZHU LJ, et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption[J]. Science, 2004, 303( 5661): 1201- 1204. DOI: 10.1126/science.1093131. [29] SHEN F, ZHENG RD, SUN XQ, et al. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease[J]. Hepatobiliary Pancreat Dis Int, 2017, 16( 4): 375- 381. DOI: 10.1016/S1499-3872(17)60019-5. [30] GUTIÉRREZ-JUÁREZ R, POCAI A, MULAS C, et al. Critical role of stearoyl-CoA desaturase-1(SCD1) in the onset of diet-induced hepatic insulin resistance[J]. J Clin Invest, 2006, 116( 6): 1686- 1695. DOI: 10.1172/JCI26991. -



 PDF下载 ( 40558 KB)
PDF下载 ( 40558 KB)

 下载:
下载: