不同自身抗体阳性的原发性胆汁性胆管炎患者预后差异及影响因素分析
DOI: 10.12449/JCH251117
Prognostic differences between primary biliary cholangitis patients positive for different autoantibodies and related influencing factors
-
摘要:
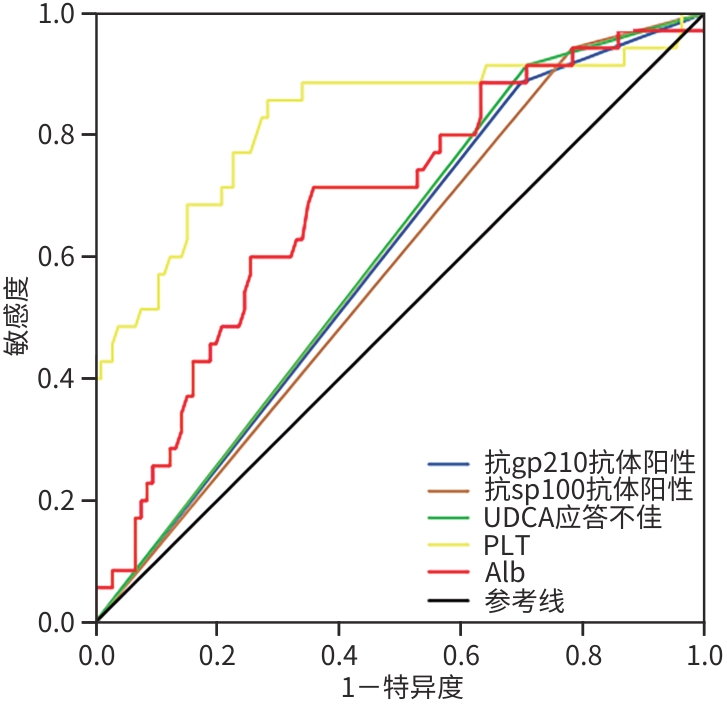
目的 探讨不同自身抗体阳性的原发性胆汁性胆管炎(PBC)患者预后差异及不良预后危险因素,以实现对PBC患者进行早期有效干预。 方法 选取2018年1月—2023年12月于山西省汾阳医院首次确诊为PBC的患者141例,分为抗线粒体抗体M2亚型(AMA-M2)单阳性组(A组)80例、AMA-M2与抗gp210抗体双阳性组(B组)36例、AMA-M2与抗sp100抗体双阳性组(C组)25例,比较3组间的一般资料、实验室指标及预后差异。预后评价采用Globe评分,Globe评分<0.3且确诊时无肝硬化为预后良好,Globe评分≥0.3或确诊时合并肝硬化则为预后不良。正态分布的计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验;非正态分布的计量资料多组间比较采用Kruskal-Wallis H检验,进一步两两比较采用Dunn’s多重检验。计数资料组间比较采用χ2检验。采用单因素和多因素Logistic回归分析影响PBC患者的预后因素,并绘制受试者操作特征曲线(ROC曲线),计算曲线下面积(AUC)。 结果 B、C组男性患者占比、肝硬化检出率、ALT、TBil和ALP水平均高于A组,PLT和Alb水平均低于A组,差异均有统计学意义(P值均<0.05)。基于熊去氧胆酸(UDCA)治疗1年后的相关指标计算Globe评分,结果显示,3组患者预后存在显著差异(P<0.001),其中B、C组Globe评分≥0.3的患者比例均显著高于A组(P值均<0.05);UDCA应答效果分析表明,B、C组应答不佳率明显高于A组(P值均<0.05)。单因素Logistic回归分析结果显示,抗gp210抗体、抗sp100抗体、UDCA应答效果、PLT、Alb、ALT、TBil和ALP与PBC患者预后相关(P值均<0.05)。将符合条件的变量均纳入多因素Logistic回归分析,结果显示,抗gp210抗体(OR=4.959,95%CI:1.112~22.122,P=0.036)、抗sp100抗体(OR=21.666,95%CI:1.542~304.449,P=0.023)、Alb(OR=0.899,95%CI:0.814~0.994,P=0.038)、PLT(OR=0.974,95%CI:0.963~0.985,P<0.001)及UDCA应答效果(OR=10.275,95%CI:1.047~100.831,P=0.046)是PBC患者预后的独立影响因素。ROC曲线结果显示,PLT对PBC患者预后预测效能最佳(AUC=0.824),敏感度和特异度分别为85.7%和71.7%。 结论 AMA-M2与抗gp210抗体双阳性和AMA-M2与抗sp100抗体双阳性的PBC患者预后更差,且UDCA应答不佳率更高。此外,抗gp210抗体阳性、抗sp100抗体阳性、血小板降低、低白蛋白血症及UDCA应答不佳均提示不良临床预后。 Abstract:Objective To investigate the prognostic differences between primary biliary cholangitis (PBC) patients positive for different autoantibodies and the risk factors for poor prognosis, and to facilitate early and effective intervention for PBC patients. Methods A total of 141 patients who were diagnosed with PBC for the first time in Fenyang Hospital of Shanxi Province from January 2018 to December 2023 were enrolled and divided into group A (80 patients positive for anti-mitochondrial antibody M2 [AMA-M2] alone), group B (36 patients positive for AMA-M2 and anti-gp210 antibody), and group C (25 patients positive for AMA-M2 and anti-sp100 antibody), and the three groups were compared in terms of general information, laboratory markers, and prognosis. The Globe score was used for prognostic evaluation, and a Globe score of<0.3 and the absence of liver cirrhosis at the time of confirmed diagnosis were defined as good prognosis, while a Globe score of ≥0.3 or the presence of liver cirrhosis at the time of confirmed diagnosis were defined as poor prognosis. A one-way analysis of variance was used for comparison of normally distributed continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups; the Kruskal-Wallis H test was used for comparison of non-normally distributed continuous data between multiple groups, and the Dunn’s multiple test was used for further comparison between two groups; the chi-square test was used for comparison of categorical data between groups. The univariate and multivariate Logistic regression analyses were used to investigate the influencing factors for the prognosis of PBC patients; the receiver operating characteristic (ROC) curve was plotted, and the area under the ROC curve (AUC) was calculated. Results Compared with group A, groups B and C had a significantly higher proportion of male patients, a significantly higher detection rate of liver cirrhosis, significantly higher levels of ALT, TBil, and ALP, and significantly lower levels of PLT and Alb (all P<0.05). The Globe score was calculated based on related indicators after treatment with ursodeoxycholic acid (UDCA) for 1 year, and the results showed that there was a significant difference in prognosis between the three groups (P<0.001), and compared with group A, groups B and C had a significantly higher proportion of patients with a Globe score of ≥0.3 (P<0.05) and a significantly higher rate of suboptimal response to UDCA (P<0.05). The univariate Logistic regression analysis showed that anti-gp210 antibody, anti-sp100 antibody, UDCA response, PLT, Alb, ALT, TBil, and ALP were associated with the prognosis of PBC patients (all P<0.05). The variables meeting related conditions were included in the multivariate Logistic regression analysis, and the results showed that anti-gp210 antibody (odds ratio [OR]=4.959, 95% confidence interval [CI]: 1.112 — 22.122, P=0.036), anti-sp100 antibody (OR=21.666, 95%CI: 1.542 — 304.449, P=0.023), Alb (OR=0.899, 95%CI: 0.814 — 0.994, P=0.038), PLT (OR=0.974, 95%CI: 0.963 — 0.985, P<0.001), and UDCA response (OR=10.275, 95%CI: 1.047 — 100.831, P=0.046) were independent influencing factors for the prognosis of PBC patients. The ROC curve analysis showed that PLT had the best performance in predicting the prognosis of PBC patients, with an AUC of 0.824, a sensitivity of 85.7%, and a specificity of 71.7%. Conclusion Patients with dual positivity for AMA-M2 and anti-gp210 antibody, as well as those with dual positivity for AMA-M2 and anti-sp100 antibody, tend to have a poorer prognosis and a higher rate of suboptimal response to UDCA. Furthermore, positivity for anti-gp210 antibody, positivity for anti-sp100 antibody, thrombocytopenia, hypoalbuminemia, and suboptimal response to UDCA all indicate poor clinical prognosis. -
Key words:
- Primary Biliary Cholangitis /
- Autoantibodies /
- Prognosis
-
表 1 3组PBC患者基线资料比较
Table 1. Comparisons of baseline characteristics among three groups of primary biliary cholangitis patients
项目 A组(n=80) B组(n=36) C组(n=25) 统计值 P值 性别[例(%)] χ2=10.420 0.005 男 9(11.3) 13(36.1)1) 7(28.0)1) 女 71(88.8) 23(63.9) 18(72.0) 年龄(岁) 59.23±11.34 60.86±12.15 61.96±11.43 F=1.018 0.601 其他免疫性疾病[例(%)] 5(6.3) 7(19.4) 4(16.0) χ2=4.950 0.084 症状[例(%)] 乏力 6(7.5) 5(13.9) 3(12.0) χ2=1.279 0.528 皮肤瘙痒 7(8.8) 4(11.1) 6(24.0) χ2=4.219 0.121 尿黄/黄染 4(5.0) 2(5.6) 4(16.0) χ2=3.671 0.160 消化道症状 3(3.8) 1(2.8) 2(8.0) χ2=1.103 0.576 水肿/腹水 2(2.5) 3(8.3) 1(4.0) χ2=2.078 0.354 肝硬化[例(%)] 19(23.8) 17(47.2)1) 12(48.0)1) χ2=8.728 0.013 高血压[例(%)] 5(6.3) 4(11.1) 5(20.0) χ2=4.102 0.129 糖尿病[例(%)] 10(12.5) 5(13.9) 7(28.0) χ2=3.583 0.167 PLT(×109/L) 173.00(99.00~243.00) 105.50(63.75~206.25)1) 104.00(80.50~114.50)1) H=17.205 <0.001 PT(s) 12.30(11.50~13.00) 12.35(11.23~12.90) 13.50(11.15~14.55) H=2.866 0.239 Alb(g/L) 39.39±5.39 33.35±5.791) 31.07±4.811) F=30.164 <0.001 AST(U/L) 50.00(32.25~84.00) 40.50(22.25~69.75) 49.00(28.50~90.00) H=2.160 0.340 ALT(U/L) 43.50(24.25~127.23) 111.00(51.00~159.25)1) 124.00(30.50~181.50)1) H=7.072 0.029 TBil(µmol/L) 17.35(11.75~25.98) 31.55(18.15~55.83)1) 28.10(14.75~45.45)1) H=14.433 <0.001 ALP(U/L) 118.00(84.25~194.53) 233.50(124.50~348.75)1) 182.00(118.00~473.00)1) H=13.688 0.001 GGT(U/L) 136.50(78.50~221.75) 194.50(93.00~680.50) 241.00(97.50~509.50) H=4.976 0.083 IgG(g/L) 13.55(9.70~17.40) 13.30(10.65~14.88) 14.60(11.05~18.65) H=2.026 0.363 IgA(g/L) 1.84(1.56~3.47) 3.46(2.59~3.61)1) 3.28(1.49~4.80) H=11.219 0.004 IgM(g/L) 1.86(1.33~5.48) 2.57(1.89~4.10) 2.55(1.65~3.82) H=1.011 0.603 注:与A组比较,1)P<0.05。
表 2 影响PBC患者预后的单因素和多因素Logistic回归分析
Table 2. Univariate and multivariate logistic regression analysis of prognostic factors in PBC patients
项目 单因素分析 多因素分析 OR 95%CI P值 OR 95%CI P值 抗gp210抗体 3.351 1.093~10.280 0.034 4.959 1.112~22.122 0.036 抗sp100抗体 4.572 1.020~20.495 0.047 21.666 1.542~304.449 0.023 UDCA应答效果 4.409 1.257~15.470 0.021 10.275 1.047~100.831 0.046 PLT(×109/L) 0.978 0.970~0.986 <0.001 0.974 0.963~0.985 <0.001 PT(s) 1.224 0.914~1.640 0.176 Alb(g/L) 1.006 1.001~1.012 0.032 0.899 0.814~0.994 0.038 AST(U/L) 1.005 0.998~1.013 0.182 ALT(U/L) 0.908 0.849~0.971 0.005 TBil(µmol/L) 1.033 1.011~1.056 0.003 ALP(U/L) 1.004 1.001~1.008 0.015 GGT(U/L) 1.001 0.999~1.003 0.222 IgG(g/L) 1.051 0.978~1.129 0.177 IgM(g/L) 1.045 0.896~1.219 0.576 IgA(g/L) 0.965 0.830~1.122 0.646 表 3 各指标预测PBC患者预后的效能分析
Table 3. Predictive performance of indicators for prognosis in PBC patients
指标 AUC 95%CI P值 敏感度(%) 特异度(%) 抗gp210抗体阳性 0.594 0.491~0.696 0.097 88.6 30.2 抗sp100抗体阳性 0.580 0.477~0.682 0.157 94.3 21.7 UDCA应答不佳 0.603 0.503~0.704 0.067 91.4 29.2 PLT 0.824 0.731~0.918 <0.001 85.7 71.7 Alb 0.686 0.585~0.788 0.001 71.4 64.2 表 4 3组患者预后差异及UDCA应答效果比较
Table 4. Comparison of prognostic differences and ursodeoxycholic acid response outcomes
项目 A组(n=80) B组(n=36) C组(n=25) χ2值 P值 Globe评分[例(%)] 15.023 <0.001 ≥0.3 49(61.3) 32(88.9) 23(92.0) <0.3 31(38.8) 4(11.1) 2(8.0) UDCA应答效果[例(%)] 6.306 0.043 应答佳 67(83.8) 24(66.7) 16(64.0) 应答不佳 13(16.3) 12(33.3) 9(36.0) -
[1] SCHNABL B. PPAR agonists in primary biliary cholangitis[J]. N Engl J Med, 2024, 390( 9): 855- 858. DOI: 10.1056/nejme2313802. [2] Chinese Society of Hepatology, Chinese Medical Association. Guidelines on the diagnosis and management of primary biliary cholangitis(2021)[J]. J Clin Hepatol, 2022, 38( 1): 35- 41.中华医学会肝病学分会. 原发性胆汁性胆管炎的诊断和治疗指南(2021)[J]. 临床肝胆病杂志, 2022, 38( 1): 35- 41. [3] HARMS MH, van BUUREN HR, CORPECHOT C, et al. Ursodeoxycholic acid therapy and liver transplant-free survival in patients with primary biliary cholangitis[J]. J Hepatol, 2019, 71( 2): 357- 365. DOI: 10.1016/j.jhep.2019.04.001. [4] European Association for the Study of the Liver. EASL clinical practice guidelines: The diagnosis and management of patients with primary biliary cholangitis[J]. J Hepatol, 2017, 67( 1): 145- 172. DOI: 10.1016/j.jhep.2017.03.022. [5] YOU H, MA X, EFE C, et al. APASL clinical practice guidance: The diagnosis and management of patients with primary biliary cholangitis[J]. Hepatol Int, 2022, 16( 1): 1- 23. DOI: 10.1007/s12072-021-10276-6. [6] ZANDANELL S, STRASSER M, FELDMAN A, et al. Similar clinical outcome of AMA immunoblot-M2-negative compared to immunoblot-positive subjects over six years of follow-up[J]. Postgrad Med, 2021, 133( 3): 291- 298. DOI: 10.1080/00325481.2021.1885945. [7] HALDAR D, JANMOHAMED A, PLANT T, et al. Antibodies to gp210 and understanding risk in patients with primary biliary cholangitis[J]. Liver Int, 2021, 41( 3): 535- 544. DOI: 10.1111/liv.14688. [8] REIG A, NORMAN GL, GARCIA M, et al. Novel anti-hexokinase 1 antibodies are associated with poor prognosis in patients with primary biliary cholangitis[J]. Am J Gastroenterol, 2020, 115( 10): 1634- 1641. DOI: 10.14309/ajg.0000000000000690. [9] NAKAMURA M, KONDO H, MORI T, et al. Anti-gp210 and anti-centromere antibodies are different risk factors for the progression of primary biliary cirrhosis[J]. Hepatology, 2007, 45( 1): 118- 127. DOI: 10.1002/hep.21472. [10] LAMMERS WJ, HIRSCHFIELD GM, CORPECHOT C, et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy[J]. Gastroenterology, 2015, 149( 7): 1804- 1812. DOI: 10.1053/j.gastro.2015.07.061. [11] YANG F, YANG Y, WANG Q, et al. The risk predictive values of UK-PBC and GLOBE scoring system in Chinese patients with primary biliary cholangitis: The additional effect of anti-gp210[J]. Aliment Pharmacol Ther, 2017, 45( 5): 733- 743. DOI: 10.1111/apt.13927. [12] CHANG YH, WANG L, YUAN Z, et al. Application and evaluation of prognostic value of continuous scoring systems in different stages of primary biliary cholangitis patients in China[J]. Int J Dig Dis, 2019, 39( 2): 102- 110. DOI: 10.3969/j.issn.1673-534X.2019.02.008.常英昊, 王璐, 袁洲, 等. 连续预后评分系统在中国原发性胆汁性胆管炎不同分期患者中的应用及评价[J]. 国际消化病杂志, 2019, 39( 2): 102- 110. DOI: 10.3969/j.issn.1673-534X.2019.02.008. [13] LAMMERS WJ, van BUUREN HR, HIRSCHFIELD GM, et al. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: An international follow-up study[J]. Gastroenterology, 2014, 147( 6): 1338- 1349. DOI: 10.1053/j.gastro.2014.08.029. [14] HIRSCHFIELD GM, BEUERS U, KUPCINSKAS L, et al. A placebo-controlled randomised trial of budesonide for PBC following an insufficient response to UDCA[J]. J Hepatol, 2021, 74( 2): 321- 329. DOI: 10.1016/j.jhep.2020.09.011. [15] NEVENS F, ANDREONE P, MAZZELLA G, et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis[J]. N Engl J Med, 2016, 375( 7): 631- 643. DOI: 10.1056/NEJMoa1509840. [16] TANAKA A, MA X, TAKAHASHI A, et al. Primary biliary cholangitis[J]. Lancet, 2024, 404( 10457): 1053- 1066. DOI: 10.1016/S0140-6736(24)01303-5. [17] HOURI I, HIRSCHFIELD GM. Primary biliary cholangitis: Pathophysiology[J]. Clin Liver Dis, 2024, 28( 1): 79- 92. DOI: 10.1016/j.cld.2023.06.006. [18] TANAKA A. Current understanding of primary biliary cholangitis[J]. Clin Mol Hepatol, 2021, 27( 1): 1- 21. DOI: 10.3350/cmh.2020.0028. [19] SAKUGAWA H, NAKASONE H, NAKAYOSHI T, et al. Epidemiology of primary biliary cirrhosis among women with elevated gamma-glutamyl transpeptidase levels in Okinawa, Japan[J]. Hepatol Res, 2003, 26( 4): 330- 336. DOI: 10.1016/s1386-6346(03)00167-0. [20] LIU ZC, WANG ZL, ZHENG JR, et al. Prevalence of primary biliary cholangitis in the Chinese general population and its influencing factors: A systematic review[J]. J Clin Hepatol, 2023, 39( 2): 325- 332. DOI: 10.3969/j.issn.1001-5256.2023.02.011.刘智成, 王资隆, 郑佳睿, 等. 我国一般人群原发性胆汁性胆管炎患病率及其影响因素的系统综述[J]. 临床肝胆病杂志, 2023, 39( 2): 325- 332. DOI: 10.3969/j.issn.1001-5256.2023.02.011. [21] HUANG CY, LIU YM, LIU H, et al. Study of clinical characteristics in patients with gp210 antibody-positive primary biliary cholangitis[J]. Chin J Hepatol, 2022, 30( 4): 419- 425. DOI: 10.3760/cma.j.cn501113-20210501-00216.黄春洋, 刘燕敏, 刘晖, 等. 抗gp210抗体阳性原发性胆汁性胆管炎患者临床特点研究[J]. 中华肝脏病杂志, 2022, 30( 4): 419- 425. DOI: 10.3760/cma.j.cn501113-20210501-00216. [22] WANG J, XIA J, YAN XM, et al. Plateletcrit as a potential index for predicting liver fibrosis in chronic hepatitis B[J]. J Viral Hepat, 2020, 27( 6): 602- 609. DOI: 10.1111/jvh.13264. [23] LIU YW, HAN K, LIU C, et al. Clinical characteristics and prognosis of concomitant primary biliary cholangitis and autoimmune diseases: A retrospective study[J]. Can J Gastroenterol Hepatol, 2021, 2021: 5557814. DOI: 10.1155/2021/5557814. [24] HU SL, ZHAO FR, HU Q, et al. Meta-analysis assessment of GP210 and SP100 for the diagnosis of primary biliary cirrhosis[J]. PLoS One, 2014, 9( 7): e101916. DOI: 10.1371/journal.pone.0101916. [25] LINDOR KD, BOWLUS CL, BOYER J, et al. Primary biliary cholangitis: 2018 practice guidance from the American Association for the Study of Liver Diseases[J]. Hepatology, 2019, 69( 1): 394- 419. DOI: 10.1002/hep.30145. [26] MYTILINAIOU MG, MEYER W, SCHEPER T, et al. Diagnostic and clinical utility of antibodies against the nuclear body promyelocytic leukaemia and Sp100 antigens in patients with primary biliary cirrhosis[J]. Clin Chim Acta, 2012, 413( 15-16): 1211- 1216. DOI: 10.1016/j.cca.2012.03.020. [27] NAKAMURA M, TAKII Y, ITO M, et al. Increased expression of nuclear envelope gp210 antigen in small bile ducts in primary biliary cirrhosis[J]. J Autoimmun, 2006, 26( 2): 138- 145. DOI: 10.1016/j.jaut.2005.10.007. -



 PDF下载 ( 947 KB)
PDF下载 ( 947 KB)

 下载:
下载:


