乙型/丙型肝炎肝硬化失代偿期患者白蛋白水平与再代偿的关联性分析
DOI: 10.12449/JCH251119
Association between albumin and recompensation in patients with hepatitis B/C virus-related decompensated liver cirrhosis
-
摘要:
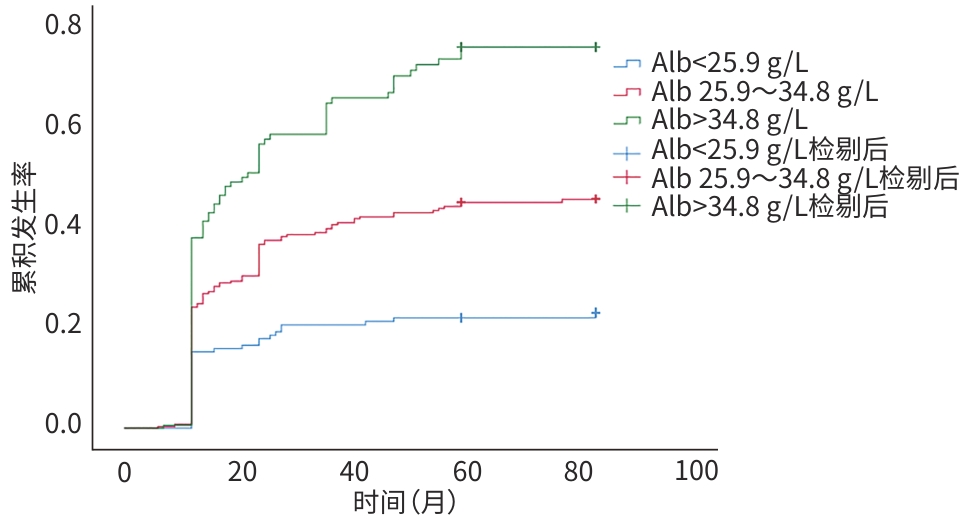
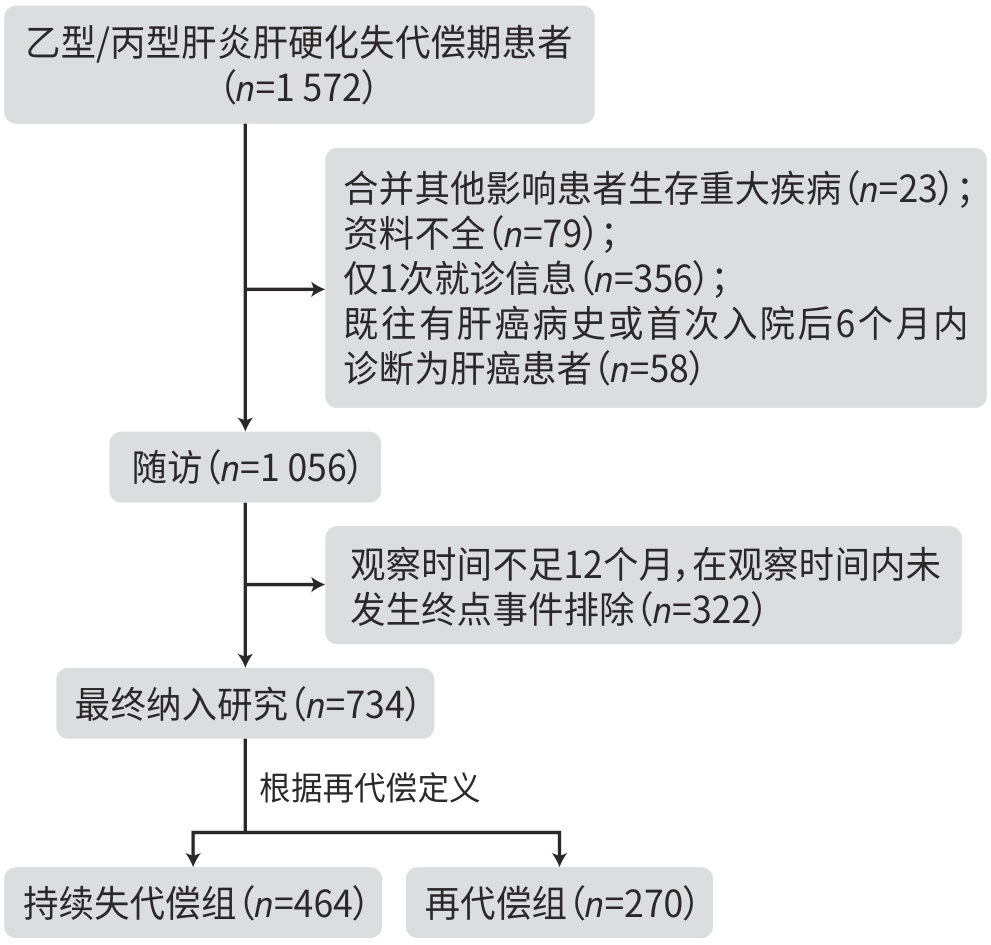
目的 通过比较不同白蛋白(Alb)水平乙型/丙型肝炎肝硬化失代偿期患者的再代偿发生率,研究Alb与再代偿的关系,为临床识别高危患者、管理患者提供指导依据。 方法 选取2016年1月1日—2022年12月31日就诊于昆明市第三人民医院的乙型及丙型肝炎肝硬化失代偿期患者734例,收集相关临床资料,根据Alb水平将所有患者分为3组,采用线性回归及χ2检验进行趋势性检验;绘制3组患者再代偿累积发生率的Kaplan-Meier曲线,并采用Log-rank检验进行比较;Cox比例风险回归模型分析Alb水平与乙型/丙型肝炎肝硬化失代偿期患者发生再代偿的关系。 结果 734例乙型/丙型肝炎肝硬化失代偿期患者中有270例发生再代偿,再代偿发生率为36.8%;所有患者入院时Alb中位水平为29.90(25.90~34.80) g/L,根据Alb水平分为3组:<25.9 g/L组(n=177)、25.9~34.8 g/L组(n=377)和>34.8 g/L组(n=180),3组患者分别有36例(20.3%)、138例(36.6%)和96例(53.3%)发生再代偿,再代偿的发生率随Alb水平升高而增高(χ2=41.730,P<0.001)。调整所有混杂因素后,与Alb<25.9 g/L组相比,Alb 25.9~34.8 g/L组和Alb>34.8 g/L组发生再代偿的HR(95%CI)分别为1.842(1.274~2.663)、2.336(1.575~3.463),呈明显上升趋势。Kaplan-Meier生存分析结果显示,3组的再代偿累积发生率差异有统计学意义(χ2=41.632,P<0.001)。 结论 Alb水平是乙型/丙型肝炎肝硬化失代偿期患者发生再代偿的影响因素,Alb水平越高,越容易发生再代偿。 Abstract:Objective To investigate the association between albumin (Alb) and recompensation by comparing recompensation rate between hepatitis B/C virus-related decompensated liver cirrhosis patients with different Alb levels, and to provide guidance for the identification and management of high-risk patients in clinical practice. Methods Related clinical data were collected from 734 patients with hepatitis B/C virus-related decompensated liver cirrhosis who attended The Third People’s Hospital of Kunming from January 1, 2016 to December 31, 2022, and they were divided into three groups based on the level of Alb. The linear regression analysis and chi-square test were used for trend tests. The Kaplan-Meier curve was plotted for the cumulative incidence rate of recompensation in the three groups, and the log-rank test was used for comparison between groups. A Cox proportional-hazards regression model analysis was used to investigate the association between Alb and recompensation in patients with hepatitis B/C virus-related decompensated liver cirrhosis. Results Among the 734 patients with hepatitis B/C virus-related decompensated liver cirrhosis, 270 achieved recompensation, with a recompensation rate of 36.8%. All patients had a median Alb level of 29.90 (25.90 — 34.80) g/L on admission, and according to the level of Alb, they were divided into <25.9 g/L group with 177 patients, 25.9 — 34.8 g/L group with 377 patients, and >34.8 g/L group with 180 patients; 36 patients (20.3%) in the <25.9 g/L group, 138 (36.6%) in the 25.9 — 34.8 g/L group, and 96 (53.3%) in the >34.8 g/L group achieved recompensation, and the recompensation rate increased with the increase in Alb level (χ2=41.730, P<0.001). After adjustment for all confounding factors, compared with the <25.9 g/L group, there was a significant increase in the incidence rate of recompensation in the 25.9 — 34.8 g/L group (hazard ratio [HR]=1.842, 95% confidence interval [CI]: 1.274 — 2.663) and the >34.8 g/L group (HR=2.336, 95% CI: 1.575 — 3.463). The Kaplan-Meier survival analysis showed that there was a significant difference in the cumulative incidence rate of recompensation between the three groups (χ2 =41.632, P<0.001). Conclusion Alb level is an influencing factor for recompensation in patients with hepatitis B/C virus-related decompensated liver cirrhosis, and the recompensation rate increases with the increase in Alb level. -
Key words:
- Hepatitis B /
- Hepatitis C /
- Liver Cirrhosis /
- Serum Albumin /
- Recompensation
-
表 1 不同Alb水平患者的基本特征
Table 1. Basic characteristics of patients with different albumin levels
项目 合计
(n=734)Alb 统计值 趋势P值 <25.9 g/L
(n=177)25.9~34.8 g/L
(n=377)>34.8 g/L
(n=180)年龄(岁) 51.21±10.10 51.48±9.99 51.55±9.68 50.23±11.02 F=1.375 0.241 男[例(%)] 517(70.4) 123(69.5) 276(73.2) 118(65.6) χ2=0.680 0.410 TIPS[例(%)] 19(2.6) 3(1.7) 9(2.4) 7(3.9) χ2=1.707 0.191 口服NSBB[例(%)] 71(9.7) 10(5.6) 40(10.6) 21(11.7) χ2=3.672 0.055 SVR[例(%)] 647(88.1) 148(83.6) 335(88.9) 164(91.1) χ2=4.776 0.029 腹水分级[例(%)] χ2=47.424 <0.001 无 62(8.4) 10(5.6) 26(6.9) 26(14.4) 少量 345(47.0) 58(32.8) 175(46.4) 112(62.2) 中量 201(27.4) 62(35.0) 115(30.5) 24(13.3) 大量 126(17.2) 47(26.6) 61(16.2) 18(10.0) Child-Pugh分级[例(%)] χ2=258.301 <0.001 A级 114(15.5) 0(0.0) 12(3.2) 102(56.7) B级 364(49.6) 61(34.5) 236(62.6) 67(37.2) C级 256(34.9) 116(65.5) 129(34.2) 11(6.1) MELD评分[例(%)] χ2=21.859 <0.001 低危 459(62.5) 33(18.6) 115(30.5) 60(33.3) 中危 153(20.8) 38(21.5) 91(24.1) 61(33.9) 高危 122(16.6) 106(59.9) 171(45.4) 59(32.8) Hb(g/L) 117.33±29.45 111.30±28.47 115.57±28.29 126.95±30.65 F=26.169 <0.001 PLT(×109/L) 85.00±49.58 85.21±49.20 81.10±44.28 92.91±58.93 F=2.162 0.142 TBil(μmol/L) 32.05(20.70~56.35) 42.30(24.15~81.85) 34.20(21.50~54.90) 25.15(18.20~37.20) F=21.744 <0.001 PT(%) 60.46±15.51 52.77±14.39 59.59±13.63 69.83±15.60 F=126.676 <0.001 门静脉宽度(mm) 12.18±2.27 12.18±2.31 12.27±2.33 11.97±2.09 F=0.814 0.367 门静脉流速(mm) 14.29±3.09 13.94±2.94 14.18±3.09 14.89±3.17 F=8.696 0.003 再代偿[例(%)] 270(36.8) 36(20.3) 138(36.6) 96(53.3) χ2=41.730 <0.001 表 2 不同Alb水平与再代偿关联的Cox比例风险回归分析
Table 2. Cox proportional risk regression analysis of the association between different Alb levels and recompensation
Alb水平 例数 模型1 模型2 模型3 HR(95%CI) P值 HR(95%CI) P值 HR(95%CI) P值 <25.9 g/L 36 1.000 1.000 1.000 25.9~34.8 g/L 138 1.958(1.356~2.826) <0.001 1.840(1.273~2.659) 0.001 1.842(1.274~2.663) 0.001 >34.8 g/L 96 2.990(2.037~4.391) <0.001 2.730(1.856~4.017) <0.001 2.336(1.575~3.463) <0.001 注:模型1,调整年龄和性别;模型2,在模型1基础上调整TIPS手术史、口服NSBB、SVR;模型3,在模型2基础上调整Hb、门静脉宽度。
-
[1] Chinese Society of Gastroenterology, Chinese Medical Association. Chinese consensus on clinical diagnosis and therapy of liver cirrhosis[J]. Chin J Digest, 2023, 43( 4): 227- 247. DOI: 10.3760/cma.j.cn311367-2023022800093.中华医学会消化病学分会. 中国肝硬化临床诊治共识意见[J]. 中华消化杂志, 2023, 43( 4): 227- 247. DOI: 10.3760/cma.j.cn311367-20230228-00093. [2] de FRANCHIS R, BOSCH J, GARCIA-TSAO G, et al. Baveno Ⅶ-Renewing consensus in portal hypertension[J]. J Hepatol, 2022, 76( 4): 959- 974. DOI: 10.1016/j.jhep.2021.12.022. [3] Chinese Society of Hepatology, Chinese Medical Association. Guidelines on the management of ascites in cirrhosis(2023 version)[J]. Chin J Hepatol, 2023, 31( 8): 813- 826. DOI: 10.3760/cma.j.cn501113-20230719-00011.中华医学会肝病学分会. 肝硬化腹水诊疗指南(2023年版)[J]. 中华肝脏病杂志, 2023, 31( 8): 813- 826. DOI: 10.3760/cma.j.cn501113-20230719-00011. [4] BONACCI M, LONDOÑO MC, ESFORZADO N, et al. Antiviral treatment with sofosbuvir and simeprevir in a kidney transplant recipient with HCV-decompensated cirrhosis: Viral eradication and removal from the liver transplant waiting list[J]. Transpl Int, 2015, 28( 11): 1345- 1349. DOI: 10.1111/tri.12622. [5] RUIZ I, FERAY C, PAWLOTSKY JM, et al. Patient with decompensated hepatitis C virus-related cirrhosis delisted for liver transplantation after successful sofosbuvir-based treatment[J]. Liver Transpl, 2015, 21( 3): 408- 409. DOI: 10.1002/lt.24051. [6] BELLI LS, BERENGUER M, CORTESI PA, et al. Delisting of liver transplant candidates with chronic hepatitis C after viral eradication: A European study[J]. J Hepatol, 2016, 65( 3): 524- 531. DOI: 10.1016/j.jhep.2016.05.010. [7] WANG Q, ZHAO H, DENG Y, et al. Validation of Baveno VII criteria for recompensation in entecavir-treated patients with hepatitis B-related decompensated cirrhosis[J]. J Hepatol, 2022, 77( 6): 1564- 1572. DOI: 10.1016/j.jhep.2022.07.037. [8] DENG Y, KANG HY, XIANG HL, et al. Durability and on-treatment predictors of recompensation in entecavir-treated patients with hepatitis B and decompensated cirrhosis[J]. JHEP Rep, 2024, 6( 7): 101091. DOI: 10.1016/j.jhepr.2024.101091. [9] RUAN JJ, WEN SF, WANG X, et al. Influencing factors for recompensation in patients with first-time decompensated hepatitis B cirrhosis[J]. J Clin Hepatol, 2022, 38( 8): 1796- 1800. DOI: 10.3969/j.issn.1001-5256.2022.08.015.阮佳佳, 温世飞, 王霞, 等. 首次失代偿期乙型肝炎肝硬化患者获得再代偿的影响因素分析[J]. 临床肝胆病杂志, 2022, 38( 8): 1796- 1800. DOI: 10.3969/j.issn.1001-5256.2022.08.015. [10] European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis[J]. J Hepatol, 2010, 53( 3): 397- 417. DOI: 10.1016/j.jhep.2010.05.004. [11] D’AMICO G, GARCIA-TSAO G, PAGLIARO L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies[J]. J Hepatol, 2006, 44( 1): 217- 231. DOI: 10.1016/j.jhep.2005.10.013. [12] HOFER BS, SIMBRUNNER B, HARTL L, et al. Hepatic recompensation according to Baveno VII criteria is linked to a significant survival benefit in decompensated alcohol-related cirrhosis[J]. Liver Int, 2023, 43( 10): 2220- 2231. DOI: 10.1111/liv.15676. [13] WEN SF, RUAN JJ, SHEN JM, et al. Development and validation of a nomogram to predict recompensation in HBV-related cirrhosis with ascites as the single first decompensating event[J]. Scand J Gastroenterol, 2023, 58( 8): 915- 922. DOI: 10.1080/00365521.2023.2181037. [14] JALAN R, BERNARDI M. Effective albumin concentration and cirrhosis mortality: From concept to reality[J]. J Hepatol, 2013, 59( 5): 918- 920. DOI: 10.1016/j.jhep.2013.08.001. [15] RABBANI G, AHN SN. Structure, enzymatic activities, glycation and therapeutic potential of human serum albumin: A natural cargo[J]. Int J Biol Macromol, 2019, 123: 979- 990. DOI: 10.1016/j.ijbiomac.2018.11.053. [16] CASULLERAS M, FLORES-COSTA R, DURAN-GÜELL M, et al. Albumin internalizes and inhibits endosomal TLR signaling in leukocytes from patients with decompensated cirrhosis[J]. Sci Transl Med, 2020, 12( 566): eaax5135. DOI: 10.1126/scitranslmed.aax5135. [17] CARACENI P, O’BRIEN A, GINES P. Long-term albumin treatment in patients with cirrhosis and ascites[J]. J Hepatol, 2022, 76( 6): 1306- 1317. DOI: 10.1016/j.jhep.2022.03.005. [18] BAI ZH, MÉNDEZ-SÁNCHEZ N, ROMEIRO FG, et al. Use of albumin infusion for cirrhosis-related complications: An international position statement[J]. JHEP Rep, 2023, 5( 8): 100785. DOI: 10.1016/j.jhepr.2023.100785. [19] GINÈS P, KRAG A, ABRALDES JG, et al. Liver cirrhosis[J]. Lancet, 2021, 398( 10308): 1359- 1376. DOI: 10.1016/S0140-6736(21)01374-X. [20] GIANNELLI V, ROUX O, LAOUÉNAN C, et al. Impact of cardiac function, refractory ascites and beta blockers on the outcome of patients with cirrhosis listed for liver transplantation[J]. J Hepatol, 2020, 72( 3): 463- 471. DOI: 10.1016/j.jhep.2019.10.002. [21] CARACENI P, RIGGIO O, ANGELI P, et al. Long-term albumin administration in decompensated cirrhosis(ANSWER): An open-label randomised trial[J]. Lancet, 2018, 391( 10138): 2417- 2429. DOI: 10.1016/S0140-6736(18)30840-7. [22] ZHAO HN, CHEN JH, BAI ZH, et al. Effect of human albumin on oxidative stress in patient with hepatic encephalopathy due to liver cirrhosis[J]. Clin J Med Off, 2024, 52( 9): 965- 967. DOI: 10.16680/j.1671-3826.2024.09.24.赵浩南, 陈纪宏, 白朝辉, 等. 人血白蛋白对肝硬化肝性脑病患者氧化应激反应影响[J]. 临床军医杂志, 2024, 52( 9): 965- 967. DOI: 10.16680/j.1671-3826.2024.09.24. [23] BAI ZH, WANG L, LIN HY, et al. Use of human albumin administration for the prevention and treatment of hyponatremia in patients with liver cirrhosis: A systematic review and meta-analysis[J]. J Clin Med, 2022, 11( 19): 5928. DOI: 10.3390/jcm11195928. [24] LEACHE L, GUTIÉRREZ-VALENCIA M, SAIZ LC, et al. Meta-analysis: Efficacy and safety of albumin in the prevention and treatment of complications in patients with cirrhosis[J]. Aliment Pharmacol Ther, 2023, 57( 6): 620- 634. DOI: 10.1111/apt.17344. [25] ZHANG CX, CAO ZJ, XIANG XG, et al. Advances in decompensated cirrhosis treatment by human serum albumin[J]. J Shanghai Jiao Tong Univ Med Sci, 2023, 43( 1): 95- 100. DOI: 10.3969/j.issn.1674-8115.2023.01.012.张宸溪, 曹竹君, 项晓刚, 等. 人血清白蛋白治疗失代偿期肝硬化的研究进展[J]. 上海交通大学学报(医学版), 2023, 43( 1): 95- 100. DOI: 10.3969/j.issn.1674-8115.2023.01.012. [26] SORT P, NAVASA M, ARROYO V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis[J]. N Engl J Med, 1999, 341( 6): 403- 409. DOI: 10.1056/NEJM199908053410603. [27] SIMÓN-TALERO M, GARCÍA-MARTÍNEZ R, TORRENS M, et al. Effects of intravenous albumin in patients with cirrhosis and episodic hepatic encephalopathy: A randomized double-blind study[J]. J Hepatol, 2013, 59( 6): 1184- 1192. DOI: 10.1016/j.jhep.2013.07.020. [28] WANG JY, ZHANG JM. Role of human blood albumin in the reversal of decompensation to decompensation in liver cirrhosis[J]. Chin Hepatol, 2024, 29( 12): 1565- 1568. DOI: 10.3969/j.issn.1008-1704.2024.12.032.王瑾瑜, 张继明. 人血白蛋白在肝硬化失代偿逆转到再代偿中的作用[J]. 肝脏, 2024, 29( 12): 1565- 1568. DOI: 10.3969/j.issn.1008-1704.2024.12.032. -



 PDF下载 ( 767 KB)
PDF下载 ( 767 KB)

 下载:
下载: