中性粒细胞与淋巴细胞和血小板比值(NLPR)对乙型肝炎肝硬化腹水患者再代偿的预测价值及列线图模型构建
DOI: 10.12449/JCH251120
Value of neutrophil-to-lymphocyte and platelet ratio in predicting recompensation in patients with hepatitis B cirrhotic ascites and establishment of a nomogram model
-
摘要:
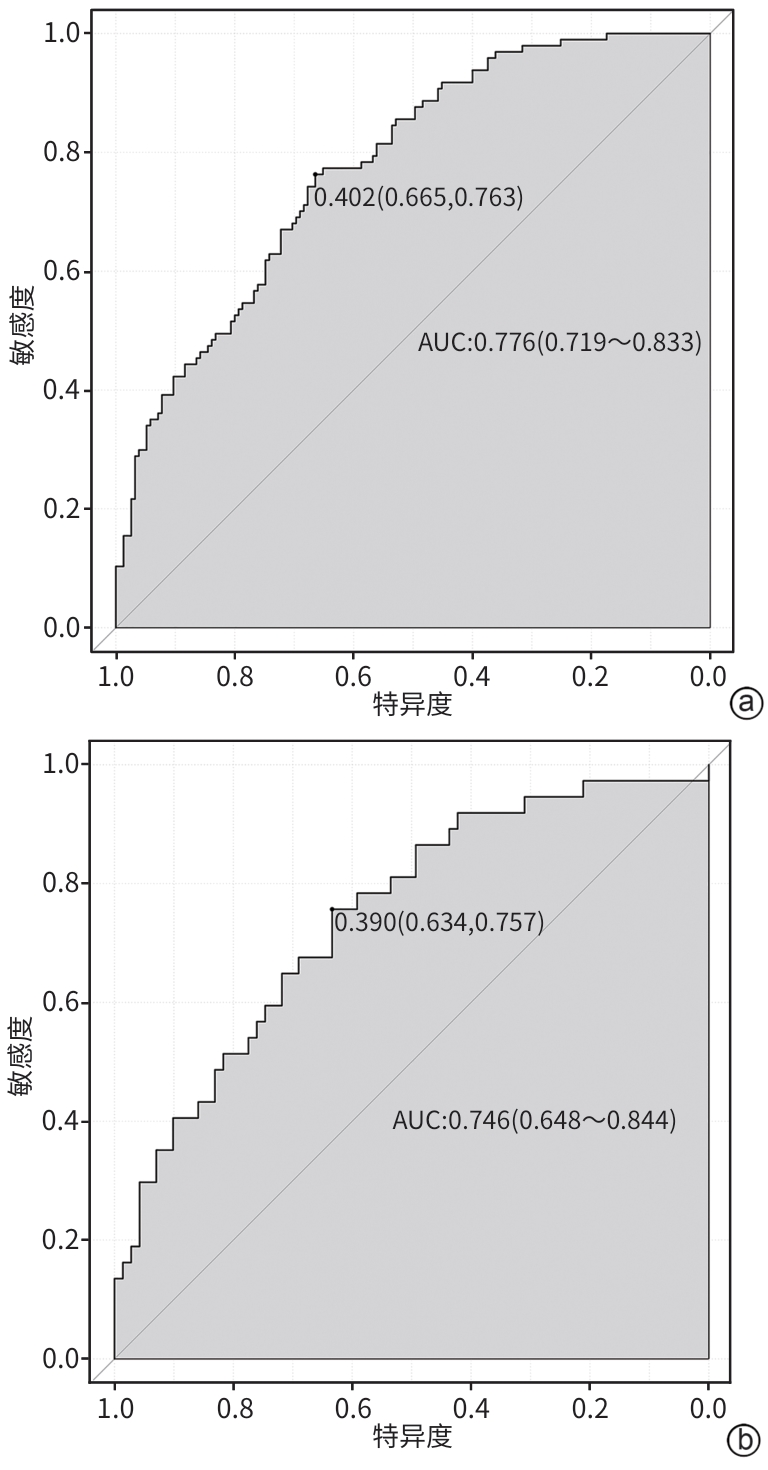
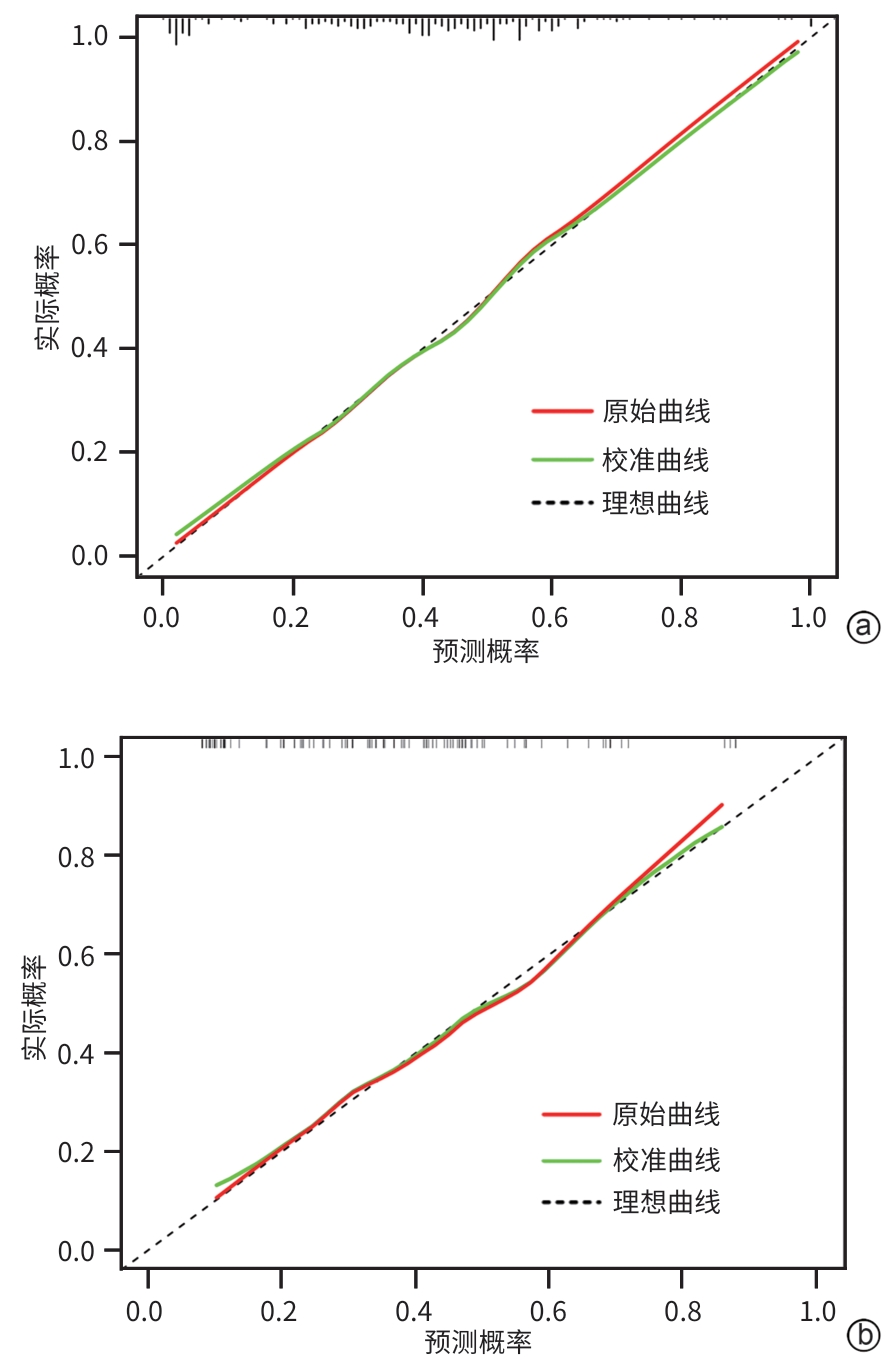
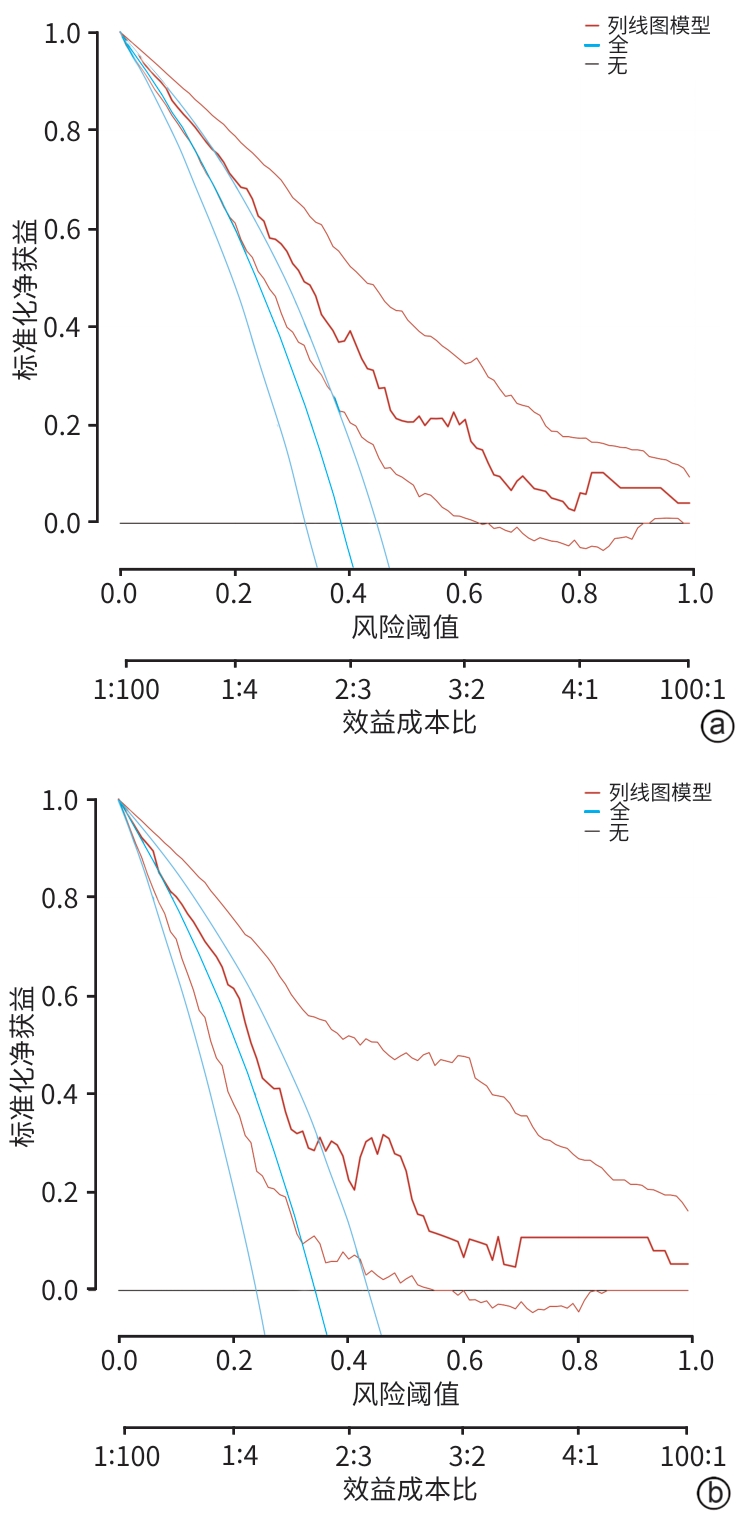
目的 探索中性粒细胞与淋巴细胞和血小板比值(NLPR)与乙型肝炎肝硬化腹水患者再代偿的关系,并构建个体化风险预测模型。 方法 选取2015年1月—2022年12月于中国人民解放军于西部战区总医院消化内科住院的乙型肝炎肝硬化腹水患者,收集患者一般资料和实验室指标,计算NLPR。计量资料两组间比较采用成组t检验或Mann-Whitney U检验;计数资料两组间比较采用χ2检验或校正χ2检验。将研究对象按7∶3比例随机分为训练集与验证集。在训练集中,通过单因素和多因素二元Logistic回归分析乙型肝炎肝硬化腹水患者再代偿的独立影响因素,构建列线图。采用受试者操作特征曲线(ROC曲线)评价新模型对乙型肝炎肝硬化腹水患者再代偿的预测价值,ROC曲线下面积(AUC)的比较采用Delong检验。绘制所构建模型的校准曲线与决策曲线,判断模型拟合程度与预测收益。 结果 本研究共纳入360例患者,其中发生再代偿患者134例。训练集患者252例,验证集患者108例,两组患者基线特征差异均无统计学意义(P值均>0.05)。Logistic回归分析发现,发生肝性脑病(OR=0.066,95%CI:0.008~0.545,P=0.012)、NLPR(OR=0.950,95%CI:0.912~0.989,P=0.012)、AFP(OR=1.012,95%CI:1.005~1.020,P<0.001)和Alb(OR=1.096,95%CI:1.031~1.166,P=0.003)是乙型肝炎肝硬化腹水患者再代偿的独立影响因素。将上述4项因素纳入列线图预测模型,训练集中列线图模型的AUC为0.776,敏感度为66.5%,特异度为76.3%,验证集中列线图模型的AUC为0.746,敏感度为63.4%,特异度为75.7%;而MELD(终末期肝病模型)评分、Child-Pugh评分、ALBI(白蛋白-胆红素)评分的AUC分别为0.574、0.628、0.621。列线图模型对乙型肝炎肝硬化腹水患者再代偿的预测效能优于其他评分(Z值分别为4.191、3.369、3.527,P值分别为<0.001、0.001、<0.001)。校准曲线与决策曲线显示该模型拟合度较好,通过此模型作出的决策可带来净收益。 结论 NLPR在预测乙型肝炎肝硬化腹水患者发生再代偿中具有一定的价值,建立的列线图模型有助于临床预测该部分患者再代偿的发生。 -
关键词:
- 乙型肝炎 /
- 肝硬化 /
- 腹水 /
- 中性粒细胞与淋巴细胞和血小板比值 /
- 列线图
Abstract:Objective To investigate the association between neutrophil-to-lymphocyte and platelet ratio (NLPR) and recompensation in patients with hepatitis B cirrhotic ascites, and to establish an individualized risk prediction model. Methods The patients with hepatitis B cirrhotic ascites who were hospitalized in Department of Gastroenterology, The General Hospital of Western Theater Command of Chinese PLA, from January 2015 to December 2022 were enrolled. General information and laboratory markers were collected, and NLPR was calculated. The independent-samples t test or the Mann-Whitney U test was used for comparison of continuous data between two groups, and the chi-square test or the chi-square test with correction was used for comparison of categorical data between two groups. The subjects were randomly divided into a training set and a validation set at a ratio of 7∶3. In the training set, univariate and multivariate binary Logistic regression analyses were used to investigate the independent influencing factors for recompensation in patients with hepatitis B cirrhotic ascites, and a nomogram was established; the receiver operating characteristic (ROC) curve was used to assess the value of the new model in predicting recompensation in patients with hepatitis B cirrhotic ascites, and the Delong test was used for comparison of the area under the ROC curve (AUC). The calibration curve and the decision curve were plotted for the model, and the model was assessed in terms of degree of fitting and predictive benefits. Results A total of 360 patients were enrolled, among whom134 achieved recompensation. There were 252 patients in the training set and 108 patients in the validation set, and there were no significant differences in baseline characteristics between the two groups (all P>0.05). The Logistic regression analysis showed that the onset of hepatic encephalopathy (odds ratio [OR]=0.066, 95% confidence interval [CI]: 0.008 — 0.545, P=0.012), NLPR (OR=0.950, 95%CI: 0.912 — 0.989, P=0.012), alpha-fetoprotein (OR=1.012, 95%CI: 1.005 — 1.020, P<0.001), and albumin (OR=1.096, 95%CI: 1.031 — 1.166, P=0.003) were independent influencing factors for recompensation in patients with hepatitis B cirrhotic ascites. The above four factors were included in a nomogram predictive model, which had an AUC of 0.776, a sensitivity of 66.5%, and a specificity of 76.3% in the training set and an AUC of 0.746, a sensitivity of 63.4%, and a specificity of 75.7% in the validation set, while Model for End-Stage Liver Disease score, Child-Pugh score, and albumin-bilirubin score had an AUC of 0.574, 0.628, and 0.621, respectively. The nomogram model had a better performance than the other three scores in predicting recompensation in patients with hepatitis B cirrhotic ascites (Z=4.191, 3.369, and 3.527, P<0.001, P=0.001, and P<0.001). The calibration curve and the decision curve showed that the model had a good degree of fitting, and the decision made using this model could bring net benefits. Conclusion NLPR has a good value in predicting recompensation in patients with hepatitis B cirrhotic ascites, and the nomogram model established can help to predict recompensation in such patients in clinical practice. -
Key words:
- Hepatitis B /
- Liver Cirrhosis /
- Ascites /
- Neutrophil to Lymphocyte and Platelet Ratio /
- Nomograms
-
表 1 不同NLPR组间的临床资料比较
Table 1. Comparison of clinical data between different NLPR groups
项目 低NLPR组(n=207) 高NLPR组(n=153) 统计值 P值 年龄(岁) 53.0(45.0~62.0) 50.0(45.0~59.8) Z=-0.835 0.403 男[例(%)] 149(71.98) 117(76.47) χ2=0.919 0.338 消化道出血[例(%)] 41(19.81) 40(26.14) χ2=2.026 0.155 肝性脑病[例(%)] 22(10.63) 31(20.26) χ2=6.503 0.011 腹水程度分级[例(%)] χ2=5.362 0.068 1级 57(27.54) 34(22.22) 2级 133(64.25) 95(62.09) 3级 17(8.21) 24(15.69) 再代偿[例(%)] 101(48.79) 33(21.57) χ2=27.903 <0.001 WBC(×109/L) 4.200(2.845~6.555) 4.695(2.963~7.133) Z=-1.900 0.057 PLT(×109/L) 73.0(53.5~102.0) 41.0(30.0~56.8) Z=-9.872 <0.001 Neu(×109/L) 2.59(1.78~4.44) 3.75(2.10~5.86) Z=-4.248 <0.001 Lym(×109/L) 0.86(0.66~1.16) 0.52(0.38~0.70) Z=-9.550 <0.001 Mono(×109/L) 0.36(0.23~0.58) 0.31(0.19~0.57) Z=-1.383 0.167 PT(s) 15.0(13.3~18.3) 15.7(14.1~20.2) Z=-2.580 0.010 INR 1.35(1.18~1.63) 1.41(1.25~1.78) Z=-2.787 0.005 AFP(ng/mL) 13.31(3.83~52.12) 5.59(2.63~18.51) Z=-3.055 0.002 Alb(g/L) 31.97±5.40 31.90±5.62 t=0.107 0.915 ALP(U/L) 132.6(94.4~179.2) 110.7(78.5~155.1) Z=-2.247 0.025 ALT(U/L) 65.2(35.4~211.0) 43.9(27.3~97.9) Z=-2.959 0.003 TBil(μmol/L) 48.7(24.9~240.1) 54.5(33.6~260.3) Z=-2.237 0.025 Scr(μmol/L) 74.0(61.1~94.2) 67.6(56.3~85.8) Z=-1.762 0.078 表 2 乙型肝炎肝硬化腹水患者训练集与验证集临床资料比较
Table 2. Comparison of clinical data between the training set and validation set
项目 训练集(n=252) 验证集(n=108) 统计值 P值 年龄(岁) 51.0(45.0~61.8) 53.0(45.0~59.0) Z=-0.008 0.993 男性[例(%)] 192(76.19) 74(68.52) χ2=2.306 0.129 消化道出血[例(%)] 62(24.60) 19(17.59) χ2=2.131 0.144 肝性脑病[例(%)] 37(14.68) 16(14.81) χ2=0.001 0.974 腹水深度分级[例(%)] χ2=2.296 0.317 1级 72(28.57) 23(21.30) 2级 146(57.94) 71(65.74) 3级 34(13.49) 14(12.96) 再代偿[例(%)] 97(38.49) 37(34.26) χ2=0.580 0.446 WBC(×109/L) 4.00(2.81~6.78) 4.48(3.01~7.55) Z=-1.549 0.121 PLT(×109/L) 58.0(41.0~86.0) 54.0(38.0~82.8) Z=-0.603 0.546 Neu(×109/L) 2.74(1.85~4.89) 3.10(1.98~5.64) Z=-1.399 0.162 Lym(×109/L) 0.72(0.50~0.99) 0.70(0.50~1.07) Z=-0.691 0.490 Mono(×109/L) 0.34(0.20~0.57) 0.35(0.24~0.58) Z=-0.930 0.353 NLPR 7.373(4.056~14.208) 7.415(4.352~13.627) Z=-0.291 0.771 PT(s) 15.40(13.70~19.08) 15.35(13.50~18.58) Z=-0.278 0.781 INR 1.39(1.23~1.71) 1.37(1.19~1.66) Z=-0.272 0.786 AFP(ng/mL) 7.43(2.99~39.61) 9.40(3.52~31.33) Z=-0.026 0.979 Alb(g/L) 31.89±5.49 32.03±5.63 t=-0.223 0.824 ALP(U/L) 122.9(90.2~169.4) 116.8(84.5~171.6) Z=-0.748 0.454 ALT(U/L) 56.0(32.4~151.8) 48.9(31.6~185.9) Z=-0.085 0.932 TBil(μmol/L) 50.9(28.4~269.7) 49.2(29.1~222.3) Z=-0.142 0.887 Scr(μmol/L) 72.4(61.0~93.0) 72.0(59.4~90.1) Z=-0.664 0.507 Child-Pugh评分(分) 9(7~12) 9(7~12) Z=-0.745 0.457 MELD评分(分) 8.947(7.108~12.945) 8.862(7.103~13.569) Z=-0.247 0.805 ALBI评分(分) -1.401(-1.944~-0.976) -1.427(-2.026~-1.059) Z=-0.222 0.825 表 3 乙型肝炎肝硬化腹水患者发生再代偿Logistic回归分析
Table 3. Logistic regression analysis of the occurrence of recompensation in patients with hepatitis B cirrhosis ascites
项目 单因素分析 多因素分析 β值 OR(95%CI) P值 β值 OR(95%CI) P值 发生肝性脑病 -3.369 0.034(0.005~0.256) <0.001 -2.719 0.066(0.008~0.545) 0.012 NLPR -0.055 0.946(0.914~0.980) 0.002 -0.052 0.950(0.912~0.989) 0.012 PT -0.094 0.910(0.859~0.964) 0.001 INR -1.081 0.339(0.177~0.649) 0.001 AFP 0.006 1.006(1.002~1.010) 0.006 0.012 1.012(1.005~1.020) <0.001 Alb 0.081 1.084(1.032~1.139) 0.001 0.092 1.096(1.031~1.166) 0.003 TBil -0.002 0.998(0.996~0.999) 0.009 -
[1] LI Z, ZHU JF, OUYANG H. Recent insights into contributing factors in the pathogenesis of cirrhotic ascites[J]. Front Med(Lausanne), 2024, 11: 1376217. DOI: 10.3389/fmed.2024.1376217. [2] D’AMICO G, PASTA L, MORABITO A, et al. Competing risks and prognostic stages of cirrhosis: A 25-year inception cohort study of 494 patients[J]. Aliment Pharmacol Ther, 2014, 39( 10): 1180- 1193. DOI: 10.1111/apt.12721. [3] de FRANCHIS R, BOSCH J, GARCIA-TSAO G, et al. Baveno VII-Renewing consensus in portal hypertension[J]. J Hepatol, 2022, 76( 4): 959- 974. DOI: 10.1016/j.jhep.2021.12.022. [4] FENG G, SONG JJ, YE F, et al. Recompensation of liver cirrhosis: Current status and challenges[J]. J Clin Hepatol, 2023, 39( 10): 2464- 2469. DOI: 10.3969/j.issn.1001-5256.2023.10.027.冯巩, 宋娟娟, 叶峰, 等. 肝硬化再代偿: 现状与挑战[J]. 临床肝胆病杂志, 2023, 39( 10): 2464- 2469. DOI: 10.3969/j.issn.1001-5256.2023.10.027. [5] SOHRABPOUR AA, MOHAMADNEJAD M, MALEKZADEH R. Review article: The reversibility of cirrhosis[J]. Aliment Pharmacol Ther, 2012, 36( 9): 824- 832. DOI: 10.1111/apt.12044. [6] TREBICKA J, AMOROS A, PITARCH C, et al. Addressing profiles of systemic inflammation across the different clinical phenotypes of acutely decompensated cirrhosis[J]. Front Immunol, 2019, 10: 476. DOI: 10.3389/fimmu.2019.00476. [7] BEDOSSA P. Reversibility of hepatitis B virus cirrhosis after therapy: Who and why?[J]. Liver Int, 2015, 35( Suppl 1): 78- 81. DOI: 10.1111/liv.12710. [8] SU X, ZHAO SG, ZHANG N. Admission NLPR predicts long-term mortality in patients with acute ischemic stroke: A retrospective analysis of the MIMIC-III database[J]. PLoS One, 2023, 18( 8): e0283356. DOI: 10.1371/journal.pone.0283356. [9] WANG HB, ZHANG R, XU J, et al. Development of a prognosis prediction model for pediatric sepsis based on the NLPR[J]. J Inflamm Res, 2024, 17: 7777- 7791. DOI: 10.2147/JIR.S479660. [10] Chinese Society of Hepatology, Chinese Medical Association. Chinese guidelines on the management of liver cirrhosis[J]. J Clin Hepatol, 2019, 35( 11): 2408- 2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006.中华医学会肝病学分会. 肝硬化诊治指南[J]. 临床肝胆病杂志, 2019, 35( 11): 2408- 2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006. [11] Chinese Society of Hepatology, Chinese Medical Association. Guidelines on the management of ascites in cirrhosis(2023 version)[J]. Chin J Hepatol, 2023, 31( 8): 813- 826. DOI: 10.3760/cma.j.cn501113-20230719-00011.中华医学会肝病学分会. 肝硬化腹水诊疗指南(2023年版)[J]. 中华肝脏病杂志, 2023, 31( 8): 813- 826. DOI: 10.3760/cma.j.cn501113-20230719-00011. [12] WANG Q, ZHAO H, DENG Y, et al. Validation of Baveno VII criteria for recompensation in entecavir-treated patients with hepatitis B-related decompensated cirrhosis[J]. J Hepatol, 2022, 77( 6): 1564- 1572. DOI: 10.1016/j.jhep.2022.07.037. [13] AITHAL GP, PALANIYAPPAN N, CHINA L, et al. Guidelines on the management of ascites in cirrhosis[J]. Gut, 2021, 70( 1): 9- 29. DOI: 10.1136/gutjnl-2020-321790. [14] AIROLA C, VARCA S, DEL GAUDIO A, et al. The covert side of ascites in cirrhosis: Cellular and molecular aspects[J]. Biomedicines, 2025, 13( 3): 680. DOI: 10.3390/biomedicines13030680. [15] TANG JJ, YAN ZJ, FENG QY, et al. The roles of neutrophils in the pathogenesis of liver diseases[J]. Front Immunol, 2021, 12: 625472. DOI: 10.3389/fimmu.2021.625472. [16] LI Q, GUO HH. Research advances in association between blood neutrophil/lymphocyte ratio and prognosis of related liver diseases[J]. J Clin Hepatol, 2017, 33( 4): 780- 784. DOI: 10.3969/j.issn.1001-5256.2017.04.041.李乔, 郭宏华. 血中性粒细胞/淋巴细胞比率与相关肝脏疾病预后关系的研究进展[J]. 临床肝胆病杂志, 2017, 33( 4): 780- 784. DOI: 10.3969/j.issn.1001-5256.2017.04.041. [17] ZHANG X, ZHANG L, TANG SH. The prognostic value of neutrophil lymphocyte ratio and its dynamic changes in the treatment of chronic hepatitis B associated acute liver failure with artificial liver[J]. J Pract Med, 2022, 38( 18): 2341- 2345. DOI: 10.3969/j.issn.1006-5725.2022.18.016.张雪, 张亮, 汤善宏. 中性粒细胞-淋巴细胞比值及其动态变化在人工肝治疗乙肝相关慢加急性肝衰竭预后的评估价值[J]. 实用医学杂志, 2022, 38( 18): 2341- 2345. DOI: 10.3969/j.issn.1006-5725.2022.18.016. [18] ZHONG LK, ZHANG G, LUO SY, et al. The value of platelet count in evaluating the degree of liver fibrosis in patients with chronic hepatitis B[J]. J Clin Lab Anal, 2020, 34( 7): e23270. DOI: 10.1002/jcla.23270. [19] HADZIYANNIS SJ, TASSOPOULOS NC, HEATHCOTE EJ, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years[J]. Gastroenterology, 2006, 131( 6): 1743- 1751. DOI: 10.1053/j.gastro.2006.09.020. [20] VARSHNEY A, GUPTA R, VERMA SK, et al. Alpha-fetoprotein as a prognostic marker in acute liver failure: A pilot study[J]. Trop Doct, 2017, 47( 3): 202- 205. DOI: 10.1177/0049475516653891. [21] WANG XP, SUN MY, YANG XJ, et al. Value of liver regeneration in predicting short-term prognosis for patients with hepatitis B-related acute-on-chronic liver failure[J]. Biomed Res Int, 2020, 2020: 5062873. DOI: 10.1155/2020/5062873. [22] BALDASSARRE M, NALDI M, ZACCHERINI G, et al. Determination of effective albumin in patients with decompensated cirrhosis: Clinical and prognostic implications[J]. Hepatology, 2021, 74( 4): 2058- 2073. DOI: 10.1002/hep.31798. -



 PDF下载 ( 2979 KB)
PDF下载 ( 2979 KB)


 下载:
下载: