丹葛解酲汤对酒精性肝病大鼠模型的改善作用及其机制分析
DOI: 10.12449/JCH251123
The mechanism of Dange Jiecheng decoction improving alcoholic liver disease in a rat model
-
摘要:
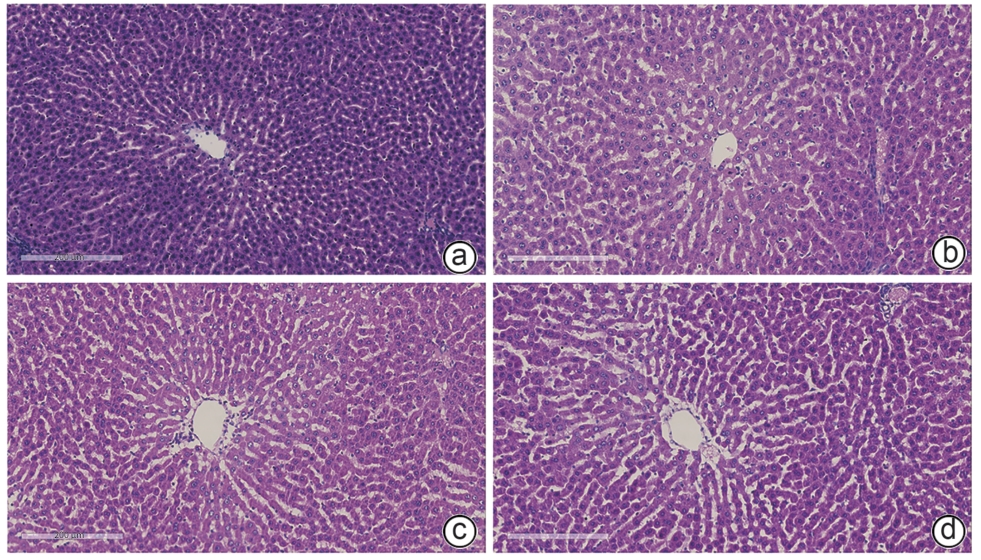
目的 探讨丹葛解酲汤对酒精性肝病(ALD)大鼠的改善作用,并基于肿瘤蛋白P53/谷胱甘肽过氧化物酶4(GPX4)信号通路分析其机制。 方法 36只雄性SD大鼠随机分为正常组、模型组、丹葛解酲汤组和复方益肝灵片组,每组9只。采用梯度灌胃56%酒精建立模型,同时灌胃给药,持续12周。实验结束收集大鼠血清和肝组织,检测大鼠血清中ALT、AST和TG水平,肝组织中丙二醛(MDA)、活性氧(ROS)、谷胱甘肽(GSH)和Fe2+水平;HE染色观察病理组织变化;油红O染色和普鲁士蓝染色分别观察肝组织脂肪及铁沉积情况;采用RT-qPCR和Western Blot法检测肝组织中P53、GPX4、NOX1、PTGS2、FTH1 mRNA和蛋白表达。计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 与正常组相比,模型组肝细胞排列紊乱,结构不完整,有炎症细胞浸润和大量脂肪空泡;血清中AST、ALT、TG和肝组织MDA、ROS、Fe2+水平均升高(P值均<0.01),P53、NOX1、PTGS2 mRNA及蛋白表达均增加(P值均<0.01),血清GSH水平及肝组织GPX4、FTH1 mRNA及蛋白表达均降低(P值均<0.01)。与模型组相比,丹葛解酲汤组和复方益肝灵片组中大鼠肝细胞炎症减少,排列整齐,血清中AST、ALT、TG和肝组织MDA、ROS、Fe2+水平及P53、NOX1、PTGS2 mRNA及蛋白表达减少(P值均<0.05),GSH水平及肝组织GPX4、FTH1 mRNA和蛋白表达水平增加(P值均<0.05),肝组织脂肪变性改善和铁沉积减少(P值均<0.01)。 结论 丹葛解酲汤可有效改善ALD大鼠疾病进展,其机制可能与控调P53/GPX4通路抑制铁死亡有关。 -
关键词:
- 肝疾病,酒精性 /
- 丹葛解酲汤 /
- 铁死亡 /
- P53/GPX4 /
- 大鼠,Sprague-Dawley
Abstract:Objective To investigate the effect of Dange Jiecheng decoction in improving alcoholic liver disease (ALD) in rats, as well as its mechanism of action based on the tumor protein P53/glutathione peroxidase 4 (GPX4) signaling pathway. Methods A total of 36 male Sprague-Dawley rats were randomly divided into normal group, model group, Dange Jiecheng decoction group, and compound Yiganling tablets group, with 9 rats in each group. The rats were given gradient intragastric administration of 56% alcohol to establish a model of ALD, and meanwhile, the corresponding drug was given by gavage for 12 consecutive weeks. After the experiment ended, serum and liver tissue samples were collected to measure the serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and triglyceride (TG) and the levels of malondialdehyde (MDA), reactive oxygen species (ROS), glutathione (GSH), and Fe2+; HE staining was used to observe histopathological changes, and oil red O staining and Prussian blue staining were used to observe fat and iron deposition in liver tissue, respectively; RT-qPCR and Western blot were used to measure the mRNA and protein expression levels of P53, glutathione peroxidase 4 (GPX4), NADPH oxidase 1 (NOX1), prostaglandin-endoperoxide synthase 2 (PTGS2), and ferritin heavy chain 1 (FTH1) in liver tissue. A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results Compared with the normal group, the model group had disordered arrangement and incomplete structure of hepatocytes with inflammatory cell infiltration and a large number of fat vacuoles, significant increases in the serum levels of AST, ALT, TG and the levels or activities of MDA, ROS, and Fe2+ in liver tissue (all P<0.01), significant increases in the mRNA and protein expression levels of P53, NOX1, and PTGS2 (all P<0.01), and significant reductions in the content of GSH in serum and the mRNA and protein expression levels of GPX4 and FTH1 in liver tissue (all P<0.01). Compared with the model group, the Dange Jiecheng decoction group and the compound Yiganling tablets group had ordered arrangement of hepatocytes with a reduction in inflammation, significant reductions in the serum levels of AST, ALT, and TG, the levels of MDA, ROS, and Fe2+ in liver tissue, and the mRNA and protein expression levels of P53, NOX1, and PTGS2 in liver tissue (all P<0.05), and significant increases in the content of GSH in serum and the mRNA and protein expression levels of GPX4 and FTH1 in liver tissue (all P<0.05), as well as an improvement in hepatic steatosis and a reduction in iron deposition (all P<0.01). Conclusion Dange Jiecheng decoction can effectively improve disease progression in rats with ALD, possibly by regulating the P53/GPX4 pathway and inhibiting ferroptosis. -
Key words:
- Liver Diseases, Alcoholic /
- Dan Ge Jie Cheng Decoction /
- Ferroptosis /
- P53/GPX4 /
- Rats, Sprague-Dawley
-
表 1 引物序列
Table 1. Primer sequences
基因 序列(5′→3′) 长度(bp) NOX1 F:TCCTAAACTACCGACTCTTCCTCA 141 R:ACTCATTGTCCCACATTGGTCTC GPX4 F:AGGCAGGAGCCAGGAAGTAATC 212 R:ACCACGCAGCCGTTCTTATC FTH1 F:CGCCAGAACTACCACCAGGACT 184 R:TCAGTTTCTCAGCATGTTCCCTC PTGS2 F:CGTTTGAAGAACTTACAGGAGAGAA 94 R:AGCAGGGCGGGATACAGTT P53 F:GGCTCCGACTATACCACTATCCACT 151 R:CACAAACACGAACCTCAAAGCT GAPDH F:CTGGAGAAACCTGCCAAGTATG 138 R:GGTGGAAGAATGGGAGTTGCT 表 2 ALD大鼠血脂和肝功能情况
Table 2. Blood lipids and liver function in rats with alcoholic liver disease
组别 动物数(只) TG(mmoL/L) ALT(U/L) AST(U/L) 正常组 6 0.04±0.01 10.24±1.35 33.23±0.92 模型组 6 2.84±0.221) 43.05±1.131) 60.99±0.511) 丹葛解酲汤组 6 1.29±0.012) 13.02±0.922) 35.93±0.872) 复方益肝灵片组 6 2.24±0.022) 34.35±2.332) 35.97±3.682) F值 356.20 440.70 132.70 P值 <0.000 1 <0.000 1 <0.000 1 注:与正常组比较,1)P<0.01;与模型组比较,2)P<0.01。
表 3 ALD大鼠肝组织MDA、ROS、GSH和Fe2+含量或活性情况
Table 3. The levels or activities of MDA, ROS, GSH, and Fe²+ in liver tissue of rats with alcoholic liver disease
组别 动物数(只) Fe2+(μmol/gprot) GSH(μmol/gprot) MDA(nmoL/mL) ROS荧光强度(%) 正常组 6 3.60±0.91 21.82±1.92 9.77±0.53 0.06±0.03 模型组 6 10.67±0.771) 6.76±1.331) 20.38±0.381) 32.75±2.191) 丹葛解酲汤组 6 5.74±0.322) 16.96±1.132) 10.77±0.382) 8.46±2.702) 复方益肝灵片组 6 7.83±0.662) 11.70±1.633) 12.44±3.362) 20.58±1.592) F值 55.63 54.03 23.52 167.30 P值 <0.000 1 <0.000 1 0.000 3 <0.000 1 注:与正常组比较,1)P<0.01;与模型组比较,2)P<0.01,3)P<0.05。
表 4 ALD大鼠肝组织脂质蓄积情况
Table 4. Lipid accumulation in liver tissue of rats with alcoholic liver disease
组别 动物数(只) 油红O染色面积(%) 正常组 6 1.05±0.11 模型组 6 6.51±0.221) 丹葛解酲汤组 6 1.77±0.112) 复方益肝灵片组 6 3.22±0.042) F值 988.70 P值 <0.000 1 注:与正常组比较,1)P<0.01;与模型组比较,2)P<0.01。
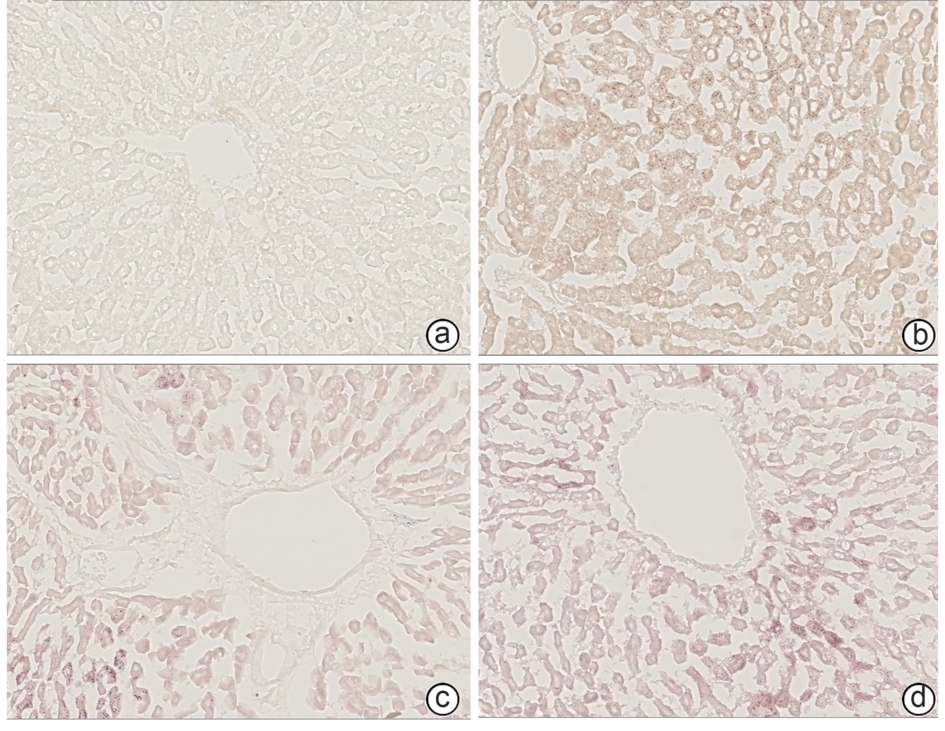
表 5 ALD大鼠肝组织铁沉积情况
Table 5. Iron deposition in liver tissue of rats with alcoholic liver disease
组别 动物数(只) 普鲁士蓝染色面积(%) 正常组 6 0.175±0.002 模型组 6 0.201±0.0031) 丹葛解酲汤组 6 0.176±0.0012) 复方益肝灵片组 6 0.186±0.0082) F值 24.06 P值 0.000 2 注:与正常组比较,1)P<0.01;与模型组比较,2)P<0.01。
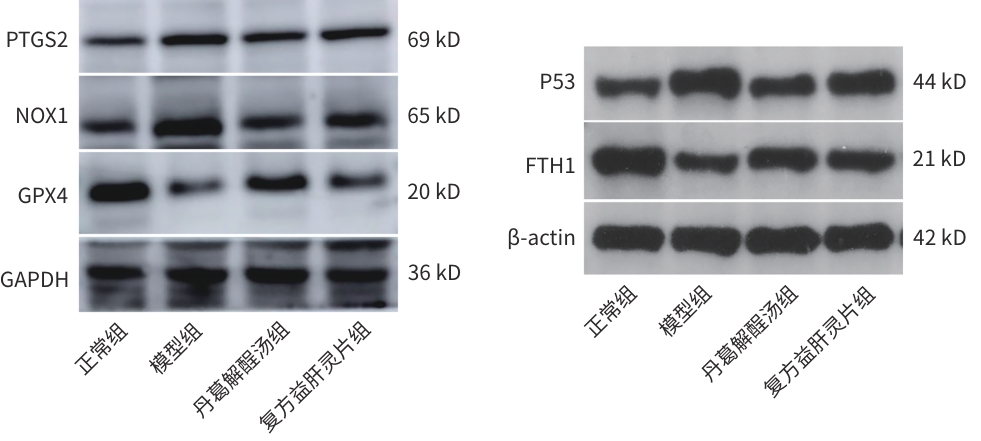
表 6 ALD大鼠P53、GPX4、NOX1、PTGS2、FTH1 mRNA相对表达量
Table 6. The mRNA expression of P53, GPX4, NOX1, PTGS2, and FTH1 in rats with alcoholic liver disease
组别 P53 mRNA NOX1 mRNA PTGS2 mRNA GPX4 mRNA FTH1 mRNA 正常组 1.02±0.24 1.01±0.13 1.01±0.17 1.01±0.13 1.01±0.13 模型组 2.17±0.291) 1.93±0.061) 1.49±0.131) 0.60±0.061) 0.62±0.051) 丹葛解酲汤组 1.34±0.252) 1.38±0.102) 1.08±0.082) 0.81±0.053) 0.83±0.023) 复方益肝灵片组 1.63±0.093) 1.67±0.073) 1.19±0.083) 0.80±0.043) 0.79±0.023) F值 13.68 54.49 8.96 13.35 15.54 P值 0.001 6 <0.000 1 0.006 2 0.001 8 0.001 1 注:与正常组比较,1)P<0.01;与模型组比较,2)P<0.01,3)P<0.05。
表 7 ALD大鼠P53、GPX4、NOX1、PTGS2、FTH1蛋白表达情况
Table 7. The protein expression of P53, GPX4, NOX1, PTGS2, and FTH1 in rats with alcoholic liver disease
组别 P53 NOX1 PTGS2 GPX4 FTH1 正常组 1.00±0.00 1.00±0.00 1.00±0.00 1.00±0.00 1.00±0.00 模型组 6.31±1.191) 1.57±0.081) 1.99±0.011) 0.49±0.011) 0.16±0.041) 丹葛解酲汤组 2.39±0.552) 1.20±0.042) 1.33±0.072) 0.72±0.032) 0.57±0.052) 复方益肝灵片组 3.83±0.622) 1.29±0.163) 1.60±0.122) 0.58±0.012) 0.34±0.063) F值 169.10 19.62 58.82 613.60 115.20 P值 <0.000 1 0.000 5 <0.000 1 <0.000 1 <0.000 1 注:与正常组比较,1)P<0.01;与模型组比较,2)P<0.01,3)P<0.05。
-
[1] WANG Q, ZHANG CR, TANG YM. Research advances in glucose and lipid metabolism disorders in different types of chronic liver diseases[J]. J Clin Hepatol, 2022, 38( 8): 1937- 1940. DOI: 10.3969/j.issn.1001-5256.2022.08.043.王倩, 张宸瑞, 唐映梅. 不同类型慢性肝病糖脂代谢紊乱研究进展[J]. 临床肝胆病杂志, 2022, 38( 8): 1937- 1940. DOI: 10.3969/j.issn.1001-5256.2022.08.043. [2] REHM J, SAMOKHVALOV AV, SHIELD KD. Global burden of alcoholic liver diseases[J]. J Hepatol, 2013, 59( 1): 160- 168. DOI: 10.1016/j.jhep.2013.03.007. [3] GAO XX, LIU LX. Progress in the epidemiology and pathogenesis of alcoholic liver diseases[J/OL]. Chin J Digest Med Imageol: Electronic Edition, 2016, 6( 2): 62- 65. DOI: 10.3877/cma.j.issn.2095-2015.2016.02.004.高潇雪, 刘立新. 酒精性肝病流行病学及发病机制研究进展[J/OL]. 中华消化病与影像杂志(电子版), 2016, 6( 2): 62- 65. DOI: 10.3877/cma.j.issn.2095-2015.2016.02.004. [4] DEVARBHAVI H, ASRANI SK, ARAB JP, et al. Global burden of liver disease: 2023 update[J]. J Hepatol, 2023, 79( 2): 516- 537. DOI: 10.1016/j.jhep.2023.03.017. [5] JIANG XJ, STOCKWELL BR, CONRAD M. Ferroptosis: Mechanisms, biology and role in disease[J]. Nat Rev Mol Cell Biol, 2021, 22( 4): 266- 282. DOI: 10.1038/s41580-020-00324-8. [6] LI X, TAO L, ZHONG MJ, et al. Ferroptosis and liver diseases[J]. J Zhejiang Univ Med Sci, 2024, 53( 6): 747- 755. DOI: 10.3724/zdxbyxb-2024-0566.李欣, 陶亮, 钟美娟, 等. 铁死亡参与肝疾病研究进展[J]. 浙江大学学报(医学版), 2024, 53( 6): 747- 755. DOI: 10.3724/zdxbyxb-2024-0566. [7] MA YD, HU XY, KE R. Research progress on treatment of alcoholic liver disease based on ferroptosis mechanism of traditional Chinese medicine[J]. J Liaoning Univ Tradit Chin Med, 2024, 26( 11): 148- 153. DOI: 10.13194/j.issn.1673-842X.2024.11.029.马伊笛, 胡晓阳, 客蕊. 中药经铁死亡途径治疗酒精性肝病研究进展[J]. 辽宁中医药大学学报, 2024, 26( 11): 148- 153. DOI: 10.13194/j.issn.1673-842X.2024.11.029. [8] CHEN X, KANG R, KROEMER G, et al. Broadening horizons: The role of ferroptosis in cancer[J]. Nat Rev Clin Oncol, 2021, 18( 5): 280- 296. DOI: 10.1038/s41571-020-00462-0. [9] SEIBT TM, PRONETH B, CONRAD M. Role of GPX4 in ferroptosis and its pharmacological implication[J]. Free Radic Biol Med, 2019, 133: 144- 152. DOI: 10.1016/j.freeradbiomed.2018.09.014. [10] JU Z, ZHANG R, YANG AG, et al. Mutant p53 promotes iron death of human colon and lung cancer cells by down-regulating glutathione peroxidase 4(GPX4) expression and increasing the production of lipid reactive oxygen species[J]. Chin J Cell Mol Immunol, 2022, 38( 6): 522- 527. DOI: 10.13423/j.cnki.cjcmi.009378.雎转, 张瑞, 杨安钢, 等. 突变型p53通过下调谷胱甘肽过氧化物酶4(GPX4)表达和增加脂质活性氧产生促进人结肠癌和肺癌细胞的铁死亡[J]. 细胞与分子免疫学杂志, 2022, 38( 6): 522- 527. DOI: 10.13423/j.cnki.cjcmi.009378. [11] CHEN C, HUANG Y, XIA PP, et al. Long noncoding RNA Meg3 mediates ferroptosis induced by oxygen and glucose deprivation combined with hyperglycemia in rat brain microvascular endothelial cells, through modulating the p53/GPX4 axis[J]. Eur J Histochem, 2021, 65( 3): 3224. DOI: 10.4081/ejh.2021.3224. [12] WANG M, MA L. Study on the mechanism of Dange Jiecheng Decoction on alcoholic liver disease based on network pharmacology and experimental verification[J]. Chin J Integr Tradit West Med Liver Dis, 2023, 33( 8): 716- 722. DOI: 10.3969/j.issn.1005-0264.2023.008.009.王铭, 马丽. 基于网络药理学与实验验证的丹葛解酲汤干预酒精性肝病机制研究[J]. 中西医结合肝病杂志, 2023, 33( 8): 716- 722. DOI: 10.3969/j.issn.1005-0264.2023.008.009. [13] WANG M, XU JH, MA L. Mechanism of action of Dange Jiecheng decoction in a rat model of alcoholic liver disease based on the Kelch-like ECH-associated protein1/nuclear factor erythroid 2-related factor 2 signaling pathway[J]. J Clin Hepatol, 2023, 39( 5): 1119- 1125. DOI: 10.3969/j.issn.1001-5256.2023.05.018.王铭, 徐建虎, 马丽. 基于Keap1/Nrf2信号通路探讨丹葛解酲汤对酒精性肝病大鼠模型的作用机制[J]. 临床肝胆病杂志, 2023, 39( 5): 1119- 1125. DOI: 10.3969/j.issn.1001-5256.2023.05.018. [14] QI LMG, ZHANG J, DUAN HJ, et al. Comparison and evaluation of methods for establishing animal models of alcoholic liver disease[J]. Pharmacol Clin Chin Mater Med, 2022, 38( 5): 153- 161. DOI: 10.13412/j.cnki.zyyl.20220627.001.其乐木格, 张娟, 段海婧, 等. 酒精性肝病动物模型建立方法的比较与评价[J]. 中药药理与临床, 2022, 38( 5): 153- 161. DOI: 10.13412/j.cnki.zyyl.20220627.001. [15] YUAN HS, PRATTE J, GIARDINA C. Ferroptosis and its potential as a therapeutic target[J]. Biochem Pharmacol, 2021, 186: 114486. DOI: 10.1016/j.bcp.2021.114486. [16] SUN YT, CHEN P, ZHAI BT, et al. The emerging role of ferroptosis in inflammation[J]. Biomed Pharmacother, 2020, 127: 110108. DOI: 10.1016/j.biopha.2020.110108. [17] ZHUANG JL, RUAN FH, QIAN TM, et al. Ferroptosis and metabolic syndrome: From basic mechanism to therapeutic strategy[J]. Chin J Evid Based Cardiovasc Med, 2025, 17( 2): 246- 251. DOI: 10.3969/j.issn.1674-4055.2025.02.23.庄金龙, 阮发晖, 钱涛铭, 等. 铁死亡与代谢综合征: 从基础机制到潜在治疗策略[J]. 中国循证心血管医学杂志, 2025, 17( 2): 246- 251. DOI: 10.3969/j.issn.1674-4055.2025.02.23. [18] LIU J, KANG R, TANG DL. Signaling pathways and defense mechanisms of ferroptosis[J]. FEBS J, 2022, 289( 22): 7038- 7050. DOI: 10.1111/febs.16059. [19] URSINI F, MAIORINO M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4[J]. Free Radic Biol Med, 2020, 152: 175- 185. DOI: 10.1016/j.freeradbiomed.2020.02.027. [20] TIAN Y, LU J, HAO XQ, et al. FTH1 inhibits ferroptosis through ferritinophagy in the 6-OHDA model of Parkinson’s disease[J]. Neurotherapeutics, 2020, 17( 4): 1796- 1812. DOI: 10.1007/s13311-020-00929-z. [21] DANG YN, HE Q, YANG SY, et al. FTH1- and SAT1-induced astrocytic ferroptosis is involved in Alzheimer’s disease: Evidence from single-cell transcriptomic analysis[J]. Pharmaceuticals(Basel), 2022, 15( 10): 1177. DOI: 10.3390/ph15101177. [22] XU R, WANG WN, ZHANG WL. Ferroptosis and the bidirectional regulatory factor p53[J]. Cell Death Discov, 2023, 9( 1): 197. DOI: 10.1038/s41420-023-01517-8. [23] ZHANG W, LIU Y, LIAO Y, et al. GPX4, ferroptosis, and diseases[J]. Biomed Pharmacother, 2024, 174: 116512. DOI: 10.1016/j.biopha.2024.116512. [24] ZHANG ZL, LIN XY, WANG WR, et al. Effect of modified Shaofu Zhuyutang on ferroptosis in ectopic endometrial tissues of rats with endometriosis based on MDM4/p53/GPX4 signaling pathway[J]. Chin J Exp Tradit Med Formulae, 2025, 31( 4): 39- 47. DOI: 10.13422/j.cnki.syfjx.20242245.张作良, 林祥羽, 王婉润, 等. 基于MDM4/p53/GPX4信号通路探讨加味少腹逐瘀汤对子宫内膜异位症大鼠异位子宫内膜组织铁死亡的影响[J]. 中国实验方剂学杂志, 2025, 31( 4): 39- 47. DOI: 10.13422/j.cnki.syfjx.20242245. [25] ZHANG JH, HUO H, XU RQ, et al. Effect of MDM2 on ferroptosis in colon cancer cells through the P53/GPX4 pathway[J/OL]. J Yunnan Minzu Univ Nat Sci Ed, 2025.[ Epub ahead of print]张金华, 霍虹, 许瑞琪, 等. MDM2通过P53/GPX4通路对结肠癌细胞铁死亡的影响[J/OL]. 云南民族大学学报(自然科学版), 2025.[网络首发] [26] GONG W, WANG Y, LI Q, et al. Regulation of SLC7A11 by LncRNA GPRC5D-AS1 mediates ferroptosis in skeletal muscle: Mechanistic exploration of sarcopenia[J]. Front Mol Biosci, 2025, 12: 1557218. DOI: 10.3389/fmolb.2025.1557218. [27] YU XY, WU WW, HAO JJ, et al. Ginger protects against vein graft remodeling by precisely modulating ferroptotic stress in vascular smooth muscle cell dedifferentiation[J]. J Pharm Anal, 2025, 15( 2): 101053. DOI: 10.1016/j.jpha.2024.101053. [28] ZHOU YQ, ZHOU HX, HUA L, et al. Verification of ferroptosis and pyroptosis and identification of PTGS2 as the hub gene in human coronary artery atherosclerosis[J]. Free Radic Biol Med, 2021, 171: 55- 68. DOI: 10.1016/j.freeradbiomed.2021.05.009. -



 PDF下载 ( 35107 KB)
PDF下载 ( 35107 KB)

 下载:
下载: