NOD样受体蛋白3(NLRP3)炎症小体对非酒精性脂肪性肝炎发生发展的影响及中医药的干预作用
DOI: 10.12449/JCH251125
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:张金雪负责撰写论文;陈佳乐负责制图;王丹、苏李宁、赖学倩负责资料分析;陈雅洁、王淼蕾、李亚静参与收集数据,修改论文;刘俊宏负责拟定写作思路,指导撰写文章并最后定稿。
Influence of NOD-like receptor protein 3 inflammasome on the development and progression of nonalcoholic steatohepatitis and the interventional effect of traditional Chinese medicine
-
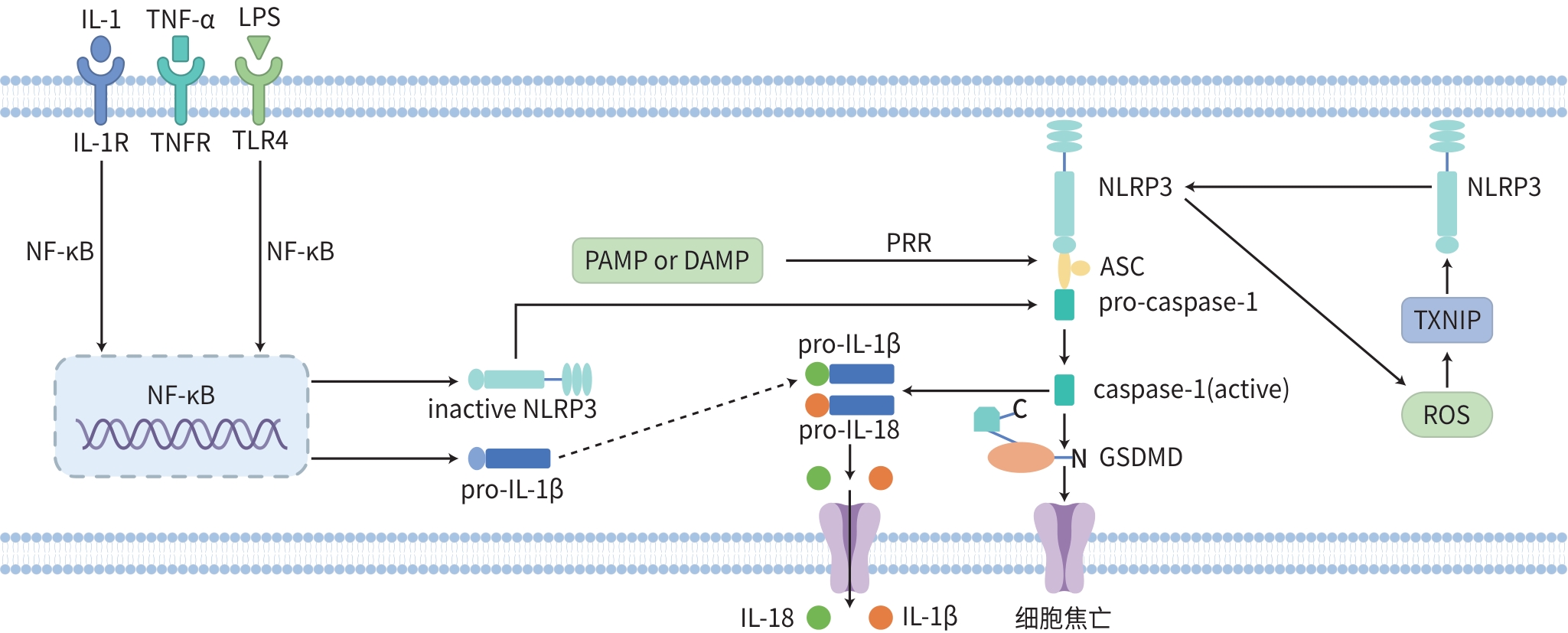
摘要: 非酒精性脂肪性肝炎(NASH)是一种以肝脂肪变性、炎性细胞浸润或伴有间质纤维增生为主要病理特征的慢性肝脏疾病,是肝纤维化、肝硬化和肝癌的重要风险阶段。NOD样受体蛋白3(NLRP3)炎症小体是先天免疫系统的核心,其异常激活与NASH的发生发展密切相关,涉及炎症反应与氧化应激等多个环节。大量研究表明,中药活性成分及中药复方可通过调节NLRP3炎症小体达到改善氧化应激、调节脂质代谢和减轻肝脏炎症的作用。临床上运用中医药治疗NASH已取得良好疗效,而炎症小体是部分中药改善NASH的关键途径或靶点之一。本文综述NLRP3炎症小体在NASH中的作用机制及中医药干预NLRP3炎症小体的研究进展,以期为NASH的中医药临床治疗提供思路,并为中药新药研发提供参考靶点及研究方向。
-
关键词:
- 非酒精性脂肪性肝病 /
- NLR家族, 热蛋白结构域包含蛋白3 /
- 中草药 /
- 药物疗法
Abstract: Nonalcoholic steatohepatitis (NASH) is a chronic liver disease with the main pathological features of hepatic steatosis, inflammatory cell infiltration, and interstitial fibroplasia, and it is an important risk factor for liver fibrosis, liver cirrhosis, and hepatocellular carcinoma. NOD-like receptor protein 3 (NLRP3) inflammasome is the core of innate immunity, and the abnormal activation of NLRP3 inflammasome is closely associated with the development and progression of NASH, which involves multiple links such as inflammatory response and oxidative stress. A large number of studies have shown that the active ingredients of traditional Chinese medicine (TCM) and TCM compound prescriptions can improve oxidative stress, regulate lipid metabolism, and alleviate liver inflammation by regulating NLRP3 inflammasome. TCM treatment applied in clinical practice has achieved a good therapeutic effect, while inflammasome is one of the key pathways or targets for TCM in improving NASH. This article reviews the mechanism of action of NLRP3 inflammasome in NASH and the research advances in TCM intervention of NLRP3 inflammasome, in order to provide ideas for the clinical TCM treatment of NASH, as well as reference targets and research directions for the research and development of new TCM drugs. -
[1] WEI SL, WANG L, EVANS PC, et al. NAFLD and NASH: Etiology, targets and emerging therapies[J]. Drug Discov Today, 2024, 29( 3): 103910. DOI: 10.1016/j.drudis.2024.103910. [2] XU XH, POULSEN KL, WU LJ, et al. Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis(NAFL/NASH)[J]. Signal Transduct Target Ther, 2022, 7( 1): 287. DOI: 10.1038/s41392-022-01119-3. [3] YOUNOSSI ZM, GOLABI P, PAIK JM, et al. The global epidemiology of nonalcoholic fatty liver disease(NAFLD) and nonalcoholic steatohepatitis(NASH): A systematic review[J]. Hepatology, 2023, 77( 4): 1335- 1347. DOI: 10.1097/HEP.0000000000000004. [4] MRIDHA AR, WREE A, ROBERTSON AAB, et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice[J]. J Hepatol, 2017, 66( 5): 1037- 1046. DOI: 10.1016/j.jhep.2017.01.022. [5] COLL RC, SCHRODER K, PELEGRÍN P. NLRP3 and pyroptosis blockers for treating inflammatory diseases[J]. Trends Pharmacol Sci, 2022, 43( 8): 653- 668. DOI: 10.1016/j.tips.2022.04.003. [6] BLEVINS HM, XU YM, BIBY S, et al. The NLRP3 inflammasome pathway: A review of mechanisms and inhibitors for the treatment of inflammatory diseases[J]. Front Aging Neurosci, 2022, 14: 879021. DOI: 10.3389/fnagi.2022.879021. [7] TAKEUCHI O, AKIRA S. Pattern recognition receptors and inflammation[J]. Cell, 2010, 140( 6): 805- 820. DOI: 10.1016/j.cell.2010.01.022. [8] FRANCHI L, EIGENBROD T, MUÑOZ-PLANILLO R, et al. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis[J]. Nat Immunol, 2009, 10( 3): 241- 247. DOI: 10.1038/ni.1703. [9] LAMKANFI M, DIXIT VM. Mechanisms and functions of inflammasomes[J]. Cell, 2014, 157( 5): 1013- 1022. DOI: 10.1016/j.cell.2014.04.007. [10] WANG LX, REN W, WU QJ, et al. NLRP3 inflammasome activation: A therapeutic target for cerebral ischemia-reperfusion injury[J]. Front Mol Neurosci, 2022, 15: 847440. DOI: 10.3389/fnmol.2022.847440. [11] FRANCHI L, WARNER N, VIANI K, et al. Function of Nod-like receptors in microbial recognition and host defense[J]. Immunol Rev, 2009, 227( 1): 106- 128. DOI: 10.1111/j.1600-065X.2008.00734.x. [12] ODURO PK, ZHENG XX, WEI JN, et al. The cGAS-STING signaling in cardiovascular and metabolic diseases: Future novel target option for pharmacotherapy[J]. Acta Pharm Sin B, 2022, 12( 1): 50- 75. DOI: 10.1016/j.apsb.2021.05.011. [13] FU JN, WU H. Structural mechanisms of NLRP3 inflammasome assembly and activation[J]. Annu Rev Immunol, 2023, 41: 301- 316. DOI: 10.1146/annurev-immunol-081022-021207. [14] QUE XY, ZHENG SH, SONG QB, et al. Fantastic voyage: The journey of NLRP3 inflammasome activation[J]. Genes Dis, 2024, 11( 2): 819- 829. DOI: 10.1016/j.gendis.2023.01.009. [15] TOURKOCHRISTOU E, AGGELETOPOULOU I, KONSTANTAKIS C, et al. Role of NLRP3 inflammasome in inflammatory bowel diseases[J]. World J Gastroenterol, 2019, 25( 33): 4796- 4804. DOI: 10.3748/wjg.v25.i33.4796. [16] SHARIF H, WANG L, WANG WL, et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome[J]. Nature, 2019, 570( 7761): 338- 343. DOI: 10.1038/s41586-019-1295-z. [17] SEOANE PI, LEE BL, HOYLE C, et al. The NLRP3-inflammasome as a sensor of organelle dysfunction[J]. J Cell Biol, 2020, 219( 12): e202006194. DOI: 10.1083/jcb.202006194. [18] COBBINA E, AKHLAGHI F. Non-alcoholic fatty liver disease(NAFLD)-pathogenesis, classification, and effect on drug metabolizing enzymes and transporters[J]. Drug Metab Rev, 2017, 49( 2): 197- 211. DOI: 10.1080/03602532.2017.1293683. [19] POUWELS S, SAKRAN N, GRAHAM Y, et al. Non-alcoholic fatty liver disease(NAFLD): A review of pathophysiology, clinical management and effects of weight loss[J]. BMC Endocr Disord, 2022, 22( 1): 63. DOI: 10.1186/s12902-022-00980-1. [20] RAMOS-TOVAR E, MURIEL P. NLRP3 inflammasome in hepatic diseases: A pharmacological target[J]. Biochem Pharmacol, 2023, 217: 115861. DOI: 10.1016/j.bcp.2023.115861. [21] SONG YJ, LIU MH, ZHAO WX. Application of pyroptosis in treating non-alcoholic steatohepatitis caused by phlegm, dampness and stasis[J]. World Chin Med, 2023, 18( 17): 2480- 2483. DOI: 10.3969/j.issn.1673-7202.2023.17.012.宋艺佳, 刘鸣昊, 赵文霞. 细胞焦亡与非酒精性脂肪性肝炎"痰湿瘀"病机的微观联系与治疗[J]. 世界中医药, 2023, 18( 17): 2480- 2483. DOI: 10.3969/j.issn.1673-7202.2023.17.012. [22] HSU SK, LI CY, LIN IL, et al. Inflammation-related pyroptosis, a novel programmed cell death pathway, and its crosstalk with immune therapy in cancer treatment[J]. Theranostics, 2021, 11( 18): 8813- 8835. DOI: 10.7150/thno.62521. [23] LI SS, SUN YM, SONG MM, et al. NLRP3/caspase-1/GSDMD-mediated pyroptosis exerts a crucial role in astrocyte pathological injury in mouse model of depression[J]. JCI Insight, 2021, 6( 23): e146852. DOI: 10.1172/jci.insight.146852. [24] PAN XS. METTL3 promotes macrophage pyroptosis in alcoholic steatohepatitis via miR-34a-5p/SIRT1 axis[D]. Hefei: Anhui Medical University, 2021.潘学胜. METTL3通过miR-34a-5p/SIRT1轴在酒精性脂肪性肝炎中促进巨噬细胞焦亡[D]. 合肥: 安徽医科大学, 2021. [25] HOU YW, ZHANG RJ, JI LS, et al. Protective effect of Zhizi Dahuang decoction in a mouse model of alcoholic liver disease[J]. J Clin Hepatol, 2023, 39( 12): 2873- 2884. DOI: 10.3969/j.issn.1001-5256.2023.12.019.侯逸文, 张荣杰, 纪龙珊, 等. 栀子大黄汤在酒精性肝病小鼠模型中的保护作用[J]. 临床肝胆病杂志, 2023, 39( 12): 2873- 2884. DOI: 10.3969/j.issn.1001-5256.2023.12.019. [26] JIN YY, SHI JW, CHEN JJ, et al. Effects of Jianpi liqi Huashi prescription on hepatocellular damage, oxidative stress and nitrative stress in mice with non-alcoholic steatohepatitis[J]. Chin J Inf Tradit Chin Med, 2024, 31( 4): 94- 99. DOI: 10.19879/j.cnki.1005-5304.202309151.金源源, 石杰文, 陈建杰, 等. 健脾理气化湿方对非酒精性脂肪性肝炎小鼠肝细胞损伤、氧化应激和硝化应激的影响[J]. 中国中医药信息杂志, 2024, 31( 4): 94- 99. DOI: 10.19879/j.cnki.1005-5304.202309151. [27] SUN LB, MA W, GAO WL, et al. Propofol directly induces caspase-1-dependent macrophage pyroptosis through the NLRP3-ASC inflammasome[J]. Cell Death Dis, 2019, 10( 8): 542. DOI: 10.1038/s41419-019-1761-4. [28] PENG ML, FU Y, WU CW, et al. Signaling pathways related to oxidative stress in diabetic cardiomyopathy[J]. Front Endocrinol, 2022, 13: 907757. DOI: 10.3389/fendo.2022.907757. [29] BAI BC, YANG YY, WANG Q, et al. NLRP3 inflammasome in endothelial dysfunction[J]. Cell Death Dis, 2020, 11: 776. DOI: 10.1038/s41419-020-02985-x. [30] ZHANG JL, ZHAO YJ, WANG SH, et al. CREBH alleviates mitochondrial oxidative stress through SIRT3 mediating deacetylation of MnSOD and suppression of Nlrp3 inflammasome in NASH[J]. Free Radic Biol Med, 2022, 190: 28- 41. DOI: 10.1016/j.freeradbiomed.2022.07.018. [31] Branch of Hepatobiliary Diseases, Chinese Association of Chinese Medicine. Diagnosis and treatment guideline for Chinese medicine on non-alcoholic steatohepatitis[J]. J Clin Hepatol, 2023, 39( 5): 1041- 1048. DOI: 10.3969/j.issn.1001-5256.2023.05.007.中华中医药学会肝胆病分会. 非酒精性脂肪性肝炎中医诊疗指南[J]. 临床肝胆病杂志, 2023, 39( 5): 1041- 1048. DOI: 10.3969/j.issn.1001-5256.2023.05.007. [32] LI KY, YANG M, ZHAO Q, et al. Discussion on TCM treatment of nonalcoholic steatohepatitis from the pathogenesis of deficiency, depression and blood stasis[J]. Chin J Integr Tradit West Med Liver Dis, 2023, 33( 8): 745- 747. DOI: 10.3969/j.issn.1005-0264.2023.008.016.李开楊, 杨梅, 赵琦, 等. 从虚、郁、瘀病机探讨非酒精性脂肪性肝炎的中医治疗[J]. 中西医结合肝病杂志, 2023, 33( 8): 745- 747. DOI: 10.3969/j.issn.1005-0264.2023.008.016. [33] LIU J, HOU K, ZHANG L. Improvement of non-alcoholic steatohepatitis by Butein and research on its mechanism[J]. Chin J Clin Pharmacol Ther, 2025, 30( 3): 355- 365. DOI: 10.12092/j.issn.1009-2501.2025.03.008.刘静, 侯凯, 张丽. 紫铆因改善非酒精性脂肪性肝炎及作用机制研究[J]. 中国临床药理学与治疗学, 2025, 30( 3): 355- 365. DOI: 10.12092/j.issn.1009-2501.2025.03.008. [34] HU QC, ZHANG WW, WU Z, et al. Baicalin and the liver-gut system: Pharmacological bases explaining its therapeutic effects[J]. Pharmacol Res, 2021, 165: 105444. DOI: 10.1016/j.phrs.2021.105444. [35] ZHANG JL, ZHANG HM, DENG XL, et al. Baicalin protects AML-12 cells from lipotoxicity via the suppression of ER stress and TXNIP/NLRP3 inflammasome activation[J]. Chem Biol Interact, 2017, 278: 189- 196. DOI: 10.1016/j.cbi.2017.10.010. [36] CHEN MY, YUE YZ, YAN S. Advances in studies on pharmacological effects and mechanisms of naringenin in treatment of digestive system diseases[J]. Chin Tradit Herb Drugs, 2024, 55( 13): 4622- 4632. DOI: 10.7501/j.issn.0253-2670.2024.13.033.陈孟瑶, 乐音子, 颜帅. 柚皮素治疗消化系统疾病的药理作用及机制研究进展[J]. 中草药, 2024, 55( 13): 4622- 4632. DOI: 10.7501/j.issn.0253-2670.2024.13.033. [37] WANG QY, OU YJ, HU GM, et al. Naringenin attenuates non-alcoholic fatty liver disease by down-regulating the NLRP3/NF-κB pathway in mice[J]. Br J Pharmacol, 2020, 177( 8): 1806- 1821. DOI: 10.1111/bph.14938. [38] WANG XH, DAI C, WANG J, et al. Therapeutic effect of neohesperidin on TNF-α-stimulated human rheumatoid arthritis fibroblast-like synoviocytes[J]. Chin J Nat Med, 2021, 19( 10): 741- 749. DOI: 10.1016/S1875-5364(21)60107-3. [39] LU YH, HUANG CY, WANG KJ, et al. Effect of neohesperidin on NLRP3/NF-κB signaling pathway on mice with non-alcoholic steatohepatitis[J]. Lishizhen Med Mater Med Res, 2024, 35( 3): 602- 607. DOI: 10.3969/j.issn.1008-0805.2024.03.21.陆一慧, 黄超原, 王恺疌, 等. 新橙皮苷对非酒精性脂肪性肝炎小鼠NLRP3/NF-κB信号通路的影响[J]. 时珍国医国药, 2024, 35( 3): 602- 607. DOI: 10.3969/j.issn.1008-0805.2024.03.21. [40] ZHAO MX, LUO B, LYU JR, et al. Effect and mechanism of cryptotanshinone on nonalcoholic fatty liver disease in mice[J]. Shaanxi Med J, 2023, 52( 9): 1135- 1139. DOI: 10.3969/j.issn.1000-7377.2023.09.005.赵梦溪, 罗斌, 吕建瑞, 等. 隐丹参酮治疗小鼠非酒精性脂肪性肝病效果及机制研究[J]. 陕西医学杂志, 2023, 52( 9): 1135- 1139. DOI: 10.3969/j.issn.1000-7377.2023.09.005. [41] LIU HB, ZHAN XY, XU G, et al. Cryptotanshinone specifically suppresses NLRP3 inflammasome activation and protects against inflammasome-mediated diseases[J]. Pharmacol Res, 2021, 164: 105384. DOI: 10.1016/j.phrs.2020.105384. [42] LIU GY, LUO LY, LI X, et al. Research progress on anti-inflammatory effects of anthraquinone compounds from rhubarb[J]. Chin Tradit Pat Med, 2023, 45( 11): 3693- 3701. DOI: 10.3969/j.issn.1001-1528.2023.11.031.刘桂元, 罗利亚, 李晓, 等. 大黄蒽醌类化合物抗炎作用研究进展[J]. 中成药, 2023, 45( 11): 3693- 3701. DOI: 10.3969/j.issn.1001-1528.2023.11.031. [43] WU C, BIAN YQ, LU BJ, et al. Rhubarb free anthraquinones improved mice nonalcoholic fatty liver disease by inhibiting NLRP3 inflammasome[J]. J Transl Med, 2022, 20( 1): 294. DOI: 10.1186/s12967-022-03495-4. [44] WANG J, ZHANG JW, ZHANG YR, et al. Syntheses and enhancing effect evaluation of the aromatic volatile oil stearate[J]. Mod Tradit Chin Med Mater Med World Sci Technol, 2023, 25( 5): 1796- 1802. DOI: 10.11842/wst.20220304006.王晶, 张金伟, 张艺蓉, 等. 丁香酚酯及肉桂醇酯对氟比洛芬透皮吸收的影响[J]. 世界科学技术-中医药现代化, 2023, 25( 5): 1796- 1802. DOI: 10.11842/wst.20220304006. [45] DAI Y, ZHANG XM, XU Y, et al. The protective effects of cinnamyl alcohol against hepatic steatosis, oxidative and inflammatory stress in nonalcoholic fatty liver disease induced by childhood obesity[J]. Immunol Invest, 2023, 52( 8): 1008- 1022. DOI: 10.1080/08820139.2023.2280248. [46] XU ZC, ZHANG M, WANG Y, et al. Gentiopicroside ameliorates diabetic renal tubulointerstitial fibrosis via inhibiting the AT1R/CK2/NF-κB pathway[J]. Front Pharmacol, 2022, 13: 848915. DOI: 10.3389/fphar.2022.848915. [47] YONG QH, HUANG CY, CHEN BN, et al. Gentiopicroside improves NASH and liver fibrosis by suppressing TLR4 and NLRP3 signaling pathways[J]. Biomed Pharmacother, 2024, 177: 116952. DOI: 10.1016/j.biopha.2024.116952. [48] CHAN YT, WANG N, TAN HY, et al. Targeting hepatic stellate cells for the treatment of liver fibrosis by natural products: Is it the dawning of a new era?[J]. Front Pharmacol, 2020, 11: 548. DOI: 10.3389/fphar.2020.00548. [49] LOU D, FANG Q, HE YH, et al. Oxymatrine alleviates high-fat diet/streptozotocin-induced non-alcoholic fatty liver disease in C57BL/6 J mice by modulating oxidative stress, inflammation and fibrosis[J]. Biomed Pharmacother, 2024, 174: 116491. DOI: 10.1016/j.biopha.2024.116491. [50] TIAN WW, TANG BH, LIU L, et al. Research progress on curcumin improving chronic low-grade inflammation and related diseases[J]. China J Chin Mater Med, 2024, 49( 10): 2607- 2618. DOI: 10.19540/j.cnki.cjcmm.20240208.602.田韦韦, 唐碧华, 刘俐, 等. 姜黄素改善慢性低度炎症及其相关疾病研究进展[J]. 中国中药杂志, 2024, 49( 10): 2607- 2618. DOI: 10.19540/j.cnki.cjcmm.20240208.602. [51] ZHANG WJ, XIA J, WANG H, et al. Research progress in the effect of curcumin against liver injury and the underlying mechanisms[J]. Chin J Hosp Pharm, 2025, 45( 1): 99- 107. DOI: 10.13286/j.1001-5213.2025.01.15.张文君, 夏江, 王昊, 等. 姜黄素对肝损伤的改善作用及其机制研究进展[J]. 中国医院药学杂志, 2025, 45( 1): 99- 107. DOI: 10.13286/j.1001-5213.2025.01.15. [52] WANG YJ, LIU FJ, LIU MR, et al. Curcumin mitigates aflatoxin B1-induced liver injury via regulating the NLRP3 inflammasome and Nrf2 signaling pathway[J]. Food Chem Toxicol, 2022, 161: 112823. DOI: 10.1016/j.fct.2022.112823. [53] LI S, MA Y, CHEN W. Active ingredients of Erhuang Quzhi Granules for treating non-alcoholic fatty liver disease based on the NF-κB/NLRP3 pathway[J]. Fitoterapia, 2023, 171: 105704. DOI: 10.1016/j.fitote.2023.105704. [54] WU TX, LIU XD, RAN XK, et al. Discussion on the mechanism of qushi Huoxue formula in treating NASH mice based on NLRP3/caspase-1/GSDMD pathway[J]. Lishizhen Med Mater Med Res, 2024, 35( 4): 769- 773. DOI: 10.3969/j.issn.1008-0805.2024.04.01.吴铁雄, 刘旭东, 冉小柯, 等. 基于经典焦亡通路探讨祛湿活血方治疗NASH小鼠的作用机制[J]. 时珍国医国药, 2024, 35( 4): 769- 773. DOI: 10.3969/j.issn.1008-0805.2024.04.01. [55] LIU AR, LI HJ, WANG LX, et al. Study on the regulatory mechanism of Qingre Quzhuo Capsule on NF-κB/NLRP3 signaling pathway in mice with non-alcoholic steatohepatitis[J]. Tianjin J Tradit Chin Med, 2024, 41( 2): 214- 221. DOI: 10.11656/j.issn.1672-1519.2024.02.14.刘爱茹, 李华君, 王立新, 等. 清热祛浊胶囊对非酒精性脂肪肝炎小鼠NF-κB/NLRP3信号通路的调控机制研究[J]. 天津中医药, 2024, 41( 2): 214- 221. DOI: 10.11656/j.issn.1672-1519.2024.02.14. [56] SHANG DF, ZHAO CL, WANG SY, et al. Modified weijingtang regulates pyroptosis of macrophages via caspase-1/GSDMD pathway[J]. Chin J Exp Tradit Med Formulae, 2024, 30( 11): 27- 33. DOI: 10.13422/j.cnki.syfjx.20232422.尚东方, 赵晨露, 王思颖, 等. 基于Caspase-1/GSDMD通路探讨加味苇茎汤对巨噬细胞焦亡模型干预作用[J]. 中国实验方剂学杂志, 2024, 30( 11): 27- 33. DOI: 10.13422/j.cnki.syfjx.20232422. [57] YUAN W, WANG BY, YANG L, et al. Clinical effect of Qishen decoction on nonalcoholic steatohepatitis and its influence on gut microbiota[J]. Chin J Integr Tradit West Med Dig, 2021, 29( 6): 383- 391. DOI: 10.3969/j.issn.1671-038X.2021.06.02.袁维, 王炳予, 杨磊, 等. 芪参汤治疗非酒精性脂肪性肝炎的临床疗效观察及对肠道菌群的影响[J]. 中国中西医结合消化杂志, 2021, 29( 6): 383- 391. DOI: 10.3969/j.issn.1671-038X.2021.06.02. [58] GAO S, GAO JW, YANG LX, et al. Mechanism of Qishen Decoction inhibition of macrophage M1 type polarization by targeting TGR5-mediated NLRP3 inflammasome[J]. J Hainan Med Univ, 2023, 29( 20): 1531- 1538. DOI: 10.13210/j.cnki.jhmu.20230829.001.高山, 高佳炜, 杨柳欣, 等. 基于TGR5介导的NLRP3炎症小体探讨芪参汤抑制巨噬细胞M1型极化改善非酒精性脂肪性肝炎的机制[J]. 海南医学院学报, 2023, 29( 20): 1531- 1538. DOI: 10.13210/j.cnki.jhmu.20230829.001. [59] ZHOU QL, SHI AH, CHEN WH, et al. Effect of Quzhi Ruangan formula on the expression of NLRP3 inflammatory corpuscles and related factors in NASH rats[J]. China J Tradit Chin Med Pharm, 2023, 38( 4): 1828- 1832.周青丽, 石安华, 陈文慧, 等. 去脂软肝方对NASH大鼠NLRP3炎症小体及相关因子表达的影响[J]. 中华中医药杂志, 2023, 38( 4): 1828- 1832. [60] XU JY, JIANG YW, YANG LL, et al. Jiangzhi Granule alleviates lipotoxic liver injury in nonalcoholic steatohepatitis mice through regulating UCP2 and JNK/c-Jun-mediated NLRP3 inflammasome activation[J]. Acad J Shanghai Univ Tradit Chin Med, 2021, 35( 2): 43- 49. DOI: 10.16306/j.1008-861x.2021.02.009.徐娇雅, 蒋雨薇, 杨丽丽, 等. 降脂颗粒调控UCP2和JNK/c-Jun介导的NLRP3炎症小体活化减轻非酒精性脂肪性肝炎小鼠脂毒性肝损伤[J]. 上海中医药大学学报, 2021, 35( 2): 43- 49. DOI: 10.16306/j.1008-861x.2021.02.009. -



 PDF下载 ( 1454 KB)
PDF下载 ( 1454 KB)

 下载:
下载:


