Wnt信号通路调控代谢相关脂肪性肝病肝纤维化的机制及临床应用前景
DOI: 10.12449/JCH251126
Mechanism of the Wnt signaling pathway regulating hepatic fibrosis associated with metabolic dysfunction-associated fatty liver disease and its application prospects
-
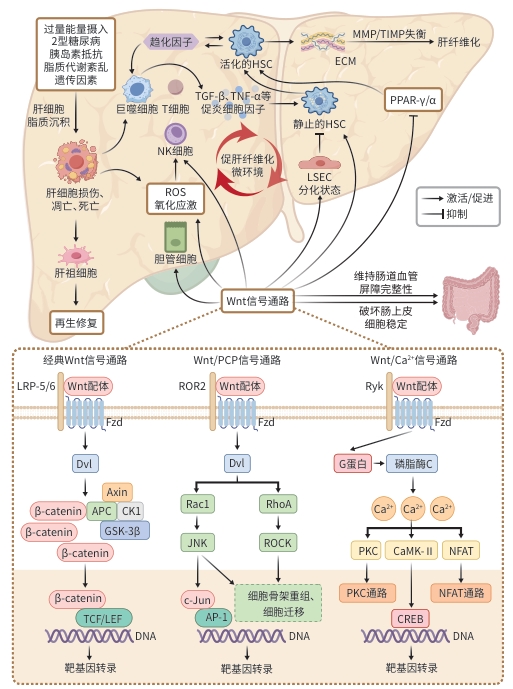
摘要: 代谢相关脂肪性肝病(MAFLD)目前已成为世界范围内需重点关注的慢性肝病之一,MAFLD患者的肝纤维化程度与其预后紧密相关,因此控制甚至逆转肝纤维化是MAFLD患者长期管理中不可忽视的部分。大量研究表明,Wnt信号通路参与MAFLD的发生发展过程。本文系统描述了Wnt信号通路,并重点阐述Wnt信号通路在MAFLD相关肝纤维化中的作用机制,为MAFLD患者肝纤维化的治疗提供参考。Abstract: Metabolic dysfunction-associated fatty liver disease (MAFLD) has become one of the critical chronic liver diseases requiring global attention, and the degree of hepatic fibrosis in these patients is closely associated with their prognosis. Therefore, control or even reversal of hepatic fibrosis is an indispensable part of the long-term management of MAFLD patients. A large number of studies have shown that the Wnt signaling pathway is involved in the development and progression of MAFLD. This article systematically describes the Wnt signaling pathway and elaborates on its mechanism of action in MAFLD-associated hepatic fibrosis, in order to provide a reference for the treatment of hepatic fibrosis in patients with MAFLD.
-
表 1 Wnt信号分子在肝纤维化中的作用
Table 1. The role of Wnt signaling molecules in liver fibrosis
Wnt信号分子 信号通路类型 作用 机制 参考文献 Wnt1 经典Wnt通路 促进 促进HSC活化、增殖和迁移,促进ECM沉积、抑制其凋亡;诱导细胞线粒
体功能障碍、过量ROS产生[17,21-22] Wnt2 经典Wnt通路 促进 活化的HSC中高表达,可能与肝纤维化相关 [9] 缓解 与HGF协同,促进肝再生;维持LSEC的分化状态,抑制HSC激活 [28-30] Wnt2b/Wnt13 经典Wnt通路 促进 促进HSC激活和ECM积累 [31] 缓解 抑制HSC活化、抑制TLR4信号通路激活,减弱HSC对TGF-β的敏感性 [32] Wnt3 经典Wnt通路 促进 可能破坏肠道上皮稳态,促进肝脏脂质沉积,间接促进肝纤维化 [34] 缓解 通过激活YAP1依赖的信号通路,促进肝细胞增殖、再生,可能缓解肝纤
维化[33] Wnt3a 经典Wnt通路 促进 促进HSC糖酵解和活化,增加ECM沉积;调节氧化应激、与PPAR相互拮
抗,间接促进肝纤维化[18,23-24] 缓解 调节hFLPC的增殖和迁移、改善大肠杆菌NF73-1引起的肠道血管内皮
屏障损伤,可能缓解肝纤维化[26-27] Wnt4 非经典Wnt通路 促进 活化的HSC中高表达,可能与肝纤维化相关 [9] Wnt5a 非经典Wnt通路 促进 促进HSC活化和增殖、ECM沉积、抑制其凋亡;增加肝内炎症,与TGF-β
协同促进肝纤维化[17] 缓解 调节hFLPC的增殖和迁移,可能缓解肝纤维化 [26] Wnt5b 非经典Wnt通路 促进 促进肝祖细胞向肌成纤维细胞的分化;可能与TGF-β协同促进HSC活化 [19,25] 缓解 可能促进胆管反应细胞分化为肝细胞,但贡献有限 [19,35] Wnt6 非经典Wnt通路 未明确 高表达可缓解肾纤维化,与肝纤维化关系尚未完全清楚 [36] Wnt9b/Wnt15 经典Wnt通路 缓解 下调可能导致LSEC毛细血管化,促进HSC活化和肝纤维化增加 [30] Wnt10a 经典Wnt通路 促进 Wnt10a的高表达促进HSC的活化和增殖 [19] 缓解 可能促进胆管反应细胞分化为肝细胞,缓解肝纤维化,但贡献有限 [19,35] Wnt10b/Wnt12 经典Wnt通路 促进 在活化HSC中上调,可能通过抑制PPAR-γ活性,维持HSC的活化表型 [20] 注:HGF,肝细胞生长因子;YAP1,Yes相关蛋白1;hFLPC,人胎儿肝祖细胞。
-
[1] ESLAM M, SANYAL AJ, GEORGE J, et al. MAFLD:A consensus-driven proposed nomenclature for metabolic associated fatty liver disease[J]. Gastroenterology, 2020, 158( 7): 1999- 2014.e1. DOI: 10.1053/j.gastro.2019.11.312. [2] ESLAM M, FAN JG, YU ML, et al. The Asian Pacific association for the study of the liver clinical practice guidelines for the diagnosis and management of metabolic dysfunction-associated fatty liver disease[J]. Hepatol Int, 2025, 19( 2): 261- 301. DOI: 10.1007/s12072-024-10774-3. [3] RINELLA ME, LAZARUS JV, RATZIU V, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature[J]. Ann Hepatol, 2024, 29( 1): 101133. DOI: 10.1016/j.aohep.2023.101133. [4] Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of metabolic dysfunction-associated(non- alcoholic)fatty liver disease(Version 2024)[J]. J Prac Hepatol, 2024, 27( 4): 494- 510. DOI: 10.3760/cma.j.cn501113-20240327-00163.中华医学会肝病学分会. 代谢相关(非酒精性)脂肪性肝病防治指南(2024年版)[J]. 实用肝脏病杂志, 2024, 27( 4): 494- 510. DOI: 10.3760/cma.j.cn501113-20240327-00163. [5] ISRAELSEN M, FRANCQUE S, TSOCHATZIS EA, et al. Steatotic liver disease[J]. Lancet, 2024, 404( 10464): 1761- 1778. DOI: 10.1016/S0140-6736(24)01811-7. [6] WU XN, XUE F, ZHANG N, et al. Global burden of liver cirrhosis and other chronic liver diseases caused by specific etiologies from 1990 to 2019[J]. BMC Public Health, 2024, 24( 1): 363. DOI: 10.1186/s12889-024-17948-6. [7] MIAO L, TARGHER G, BYRNE CD, et al. Current status and future trends of the global burden of MASLD[J]. Trends Endocrinol Metab, 2024, 35( 8): 697- 707. DOI: 10.1016/j.tem.2024.02.007. [8] SIMON TG, ROELSTRAETE B, KHALILI H, et al. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: Results from a nationwide cohort[J]. Gut, 2021, 70( 7): 1375- 1382. DOI: 10.1136/gutjnl-2020-322786. [9] SHREE HARINI K, EZHILARASAN D. Wnt/beta-catenin signaling and its modulators in nonalcoholic fatty liver diseases[J]. Hepatobiliary Pancreat Dis Int, 2023, 22( 4): 333- 345. DOI: 10.1016/j.hbpd.2022.10.003. [10] PERUGORRIA MJ, OLAIZOLA P, LABIANO I, et al. Wnt-β-catenin signalling in liver development, health and disease[J]. Nat Rev Gastroenterol Hepatol, 2019, 16( 2): 121- 136. DOI: 10.1038/s41575-018-0075-9. [11] RIM EY, CLEVERS H, NUSSE R. The Wnt pathway: From signaling mechanisms to synthetic modulators[J]. Annu Rev Biochem, 2022, 91: 571- 598. DOI: 10.1146/annurev-biochem-040320-103615. [12] QIN K, YU M, FAN JM, et al. Canonical and noncanonical Wnt signaling: Multilayered mediators, signaling mechanisms and major signaling crosstalk[J]. Genes Dis, 2023, 11( 1): 103- 134. DOI: 10.1016/j.gendis.2023.01.030. [13] ISHITANI T, KISHIDA S, HYODO-MIURA J, et al. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling[J]. Mol Cell Biol, 2003, 23( 1): 131- 139. DOI: 10.1128/MCB.23.1.131-139.2003. [14] SCHWABE RF, TABAS I, PAJVANI UB. Mechanisms of fibrosis development in nonalcoholic steatohepatitis[J]. Gastroenterology, 2020, 158( 7): 1913- 1928. DOI: 10.1053/j.gastro.2019.11.311. [15] BOUREBABA N, MARYCZ K. Hepatic stellate cells role in the course of metabolic disorders development- A molecular overview[J]. Pharmacol Res, 2021, 170: 105739. DOI: 10.1016/j.phrs.2021.105739. [16] LI YQ, TANG WJ, ZHOU YJ. Role of intestinal microbiota and metabolites in the development, progression, and treatment of nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2023, 39( 8): 1805- 1810. DOI: 10.3969/j.issn.1001-5256.2023.08.006.李永强, 唐文娟, 周永健. 肠道菌群及其代谢产物在非酒精性脂肪性肝病发生发展及治疗中的作用[J]. 临床肝胆病杂志, 2023, 39( 8): 1805- 1810. DOI: 10.3969/j.issn.1001-5256.2023.08.006. [17] DU JH, REN WG, ZHANG QS, et al. Heme oxygenase-1 suppresses Wnt signaling pathway in nonalcoholic steatohepatitis-related liver fibrosis[J]. Biomed Res Int, 2020, 2020: 4910601. DOI: 10.1155/2020/4910601. [18] WANG FX, CHEN L, KONG DS, et al. Canonical Wnt signaling promotes HSC glycolysis and liver fibrosis through an LDH-A/HIF-1α transcriptional complex[J]. Hepatology, 2024, 79( 3) 606- 623. DOI: 10.1097/HEP.0000000000000569. [19] ESMAIL MM, SAEED NM, MICHEL HE, et al. The ameliorative effect of niclosamide on bile duct ligation induced liver fibrosis via suppression of NOTCH and Wnt pathways[J]. Toxicol Lett, 2021, 347: 23- 35. DOI: 10.1016/j.toxlet.2021.04.018. [20] PERKINS RS, SINGH R, ABELL AN, et al. The role of WNT10B in physiology and disease: A 10-year update[J]. Front Cell Dev Biol, 2023, 11: 1120365. DOI: 10.3389/fcell.2023.1120365. [21] XI Y, LACANNA R, MA HY, et al. A WISP1 antibody inhibits MRTF signaling to prevent the progression of established liver fibrosis[J]. Cell Metab, 2022, 34( 9): 1377- 1393.e8. DOI: 10.1016/j.cmet.2022.07.009. [22] MIAO JH, HUANG JW, LUO CW, et al. Klotho retards renal fibrosis through targeting mitochondrial dysfunction and cellular senescence in renal tubular cells[J]. Physiol Rep, 2021, 9( 2): e14696. DOI: 10.14814/phy2.14696. [23] XU DN, ZHUANG SQ, CHEN HZ, et al. IL-33 regulates adipogenesis via Wnt/β-catenin/PPAR-γ signaling pathway in preadipocytes[J]. J Transl Med, 2024, 22( 1): 363. DOI: 10.1186/s12967-024-05180-0. [24] TABAA MM EL, FAHEEM H, ELBALLAL MS, et al. The PPAR-α agonist oleoyethanolamide(OEA) ameliorates valproic acid-induced steatohepatitis in rats via suppressing Wnt3a/β-catenin and activating PGC-1α: Involvement of network pharmacology and molecular docking[J]. Eur J Pharmacol, 2025, 991: 177306. DOI: 10.1016/j.ejphar.2025.177306. [25] MOTIZUKI M, YOKOYAMA T, SAITOH M, et al. The Snail signaling branch downstream of the TGF-β/Smad3 pathway mediates Rho activation and subsequent stress fiber formation[J]. J Biol Chem, 2024, 300( 1): 105580. DOI: 10.1016/j.jbc.2023.105580. [26] LIU ZW, KUNA VK, XU B, et al. Wnt ligands 3a and 5a regulate proliferation and migration in human fetal liver progenitor cells[J]. Transl Gastroenterol Hepatol, 2021, 6: 56. DOI: 10.21037/tgh.2020.01.12. [27] KE ZL, HUANG YB, XU J, et al. Escherichia coli NF73-1 disrupts the gut-vascular barrier and aggravates high-fat diet-induced fatty liver disease via inhibiting Wnt/β-catenin signalling pathway[J]. Liver Int, 2024, 44( 3): 776- 790. DOI: 10.1111/liv.15823. [28] DONG X, LUO Y, LU S, et al. Ursodesoxycholic acid alleviates liver fibrosis via proregeneration by activation of the ID1-WNT2/HGF signaling pathway[J]. Clin Transl Med, 2021, 11( 2): e296. DOI: 10.1002/ctm2.296. [29] CHEN T, SHI ZM, ZHAO YM, et al. LncRNA Airn maintains LSEC differentiation to alleviate liver fibrosis via the KLF2-ENOS-sGC pathway[J]. BMC Med, 2022, 20( 1): 335. DOI: 10.1186/s12916-022-02523-w. [30] DUAN JL, RUAN B, YAN XC, et al. Endothelial Notch activation reshapes the angiocrine of sinusoidal endothelia to aggravate liver fibrosis and blunt regeneration in mice[J]. Hepatology, 2018, 68( 2): 677- 690. DOI: 10.1002/hep.29834. [31] XU T, PAN LX, LI LY, et al. microRNA-708 modulates Hepatic Stellate Cells activation and enhances extracellular matrix accumulation via direct targeting TMEM88[J]. J Cell Mol Med, 2020, 24( 13): 7127- 7140. DOI: 10.1111/jcmm.15119. [32] YUAN Y, HAN QJ, LI SY, et al. Wnt2b attenuates HSCs activation and liver fibrosis through negative regulating TLR4 signaling[J]. Sci Rep, 2017, 7( 1): 3952. DOI: 10.1038/s41598-017-04374-5. [33] RIGUAL MDM, ANGULO-AGUADO M, ZAGORAC S, et al. Macrophages harness hepatocyte glutamate to boost liver regeneration[J]. Nature, 2025, 641( 8064): 1005- 1016. DOI: 10.1038/s41586-025-08778-6. [34] ZHANG P, LIU JL, LEE A, et al. IL-22 resolves MASLD via enterocyte STAT3 restoration of diet-perturbed intestinal homeostasis[J]. Cell Metab, 2024, 36( 10): 2341- 2354.e6. DOI: 10.1016/j.cmet.2024.08.012. [35] CHEN Y, GAO WK, SHU YY, et al. Mechanisms of ductular reaction in non-alcoholic steatohepatitis[J]. World J Gastroenterol, 2022, 28( 19): 2088- 2099. DOI: 10.3748/wjg.v28.i19.2088. [36] WEI M, ZHANG CM, TIAN YJ, et al. Expression and function of WNT6: From development to disease[J]. Front Cell Dev Biol, 2020, 8: 558155. DOI: 10.3389/fcell.2020.558155. [37] CATALANO T, SELVAGGI F, ESPOSITO DL, et al. Infectious agents induce Wnt/β-catenin pathway deregulation in primary liver cancers[J]. Microorganisms, 2023, 11( 7): 1632. DOI: 10.3390/microorganisms11071632. [38] SHREE HARINI K, EZHILARASAN D, MANI U. Molecular insights on intracellular Wnt/β-catenin signaling in alcoholic liver disease[J]. Cell Biochem Funct, 2024, 42( 1): e3916. DOI: 10.1002/cbf.3916. [39] LEI ZL, YANG LX, YANG YH, et al. Activation of Wnt/β-catenin pathway causes insulin resistance and increases lipogenesis in HepG2 cells via regulation of endoplasmic reticulum stress[J]. Biochem Biophys Res Commun, 2020, 526( 3): 764- 771. DOI: 10.1016/j.bbrc.2020.03.147. [40] ASTARINI F DWI, RATNASARI N, WASITYASTUTI W. Update on non-alcoholic fatty liver disease-associated single nucleotide polymorphisms and their involvement in liver steatosis, inflammation, and fibrosis: A narrative review[J]. Iran Biomed J, 2022, 26( 4): 252- 268. DOI: 10.52547/ibj.3647. [41] QIU YY, ZHANG J, ZENG FY, et al. Roles of the peroxisome proliferator-activated receptors(PPARs) in the pathogenesis of nonalcoholic fatty liver disease(NAFLD)[J]. Pharmacol Res, 2023, 192: 106786. DOI: 10.1016/j.phrs.2023.106786. [42] TERATANI T, TOMITA K, SUZUKI T, et al. Aortic carboxypeptidase-like protein, a WNT ligand, exacerbates nonalcoholic steatohepatitis[J]. J Clin Invest, 2018, 128( 4): 1581- 1596. DOI: 10.1172/JCI92863. [43] LI F, LI MW, WANG YS. Therapeutic paradigms and potential therapies for nonalcoholic steatohepatitis[J]. J Clin Hepatol, 2024, 40( 10): 2082- 2086. DOI: 10.12449/JCH241025.李凤, 李茂微, 王雨杉. 非酒精性脂肪肝病的治疗模式和潜在疗法[J]. 临床肝胆病杂志, 2024, 40( 10): 2082- 2086. DOI: 10.12449/JCH241025. [44] EL-DERANY MO, EL-DEMERDASH E. Pyrvinium pamoate attenuates non-alcoholic steatohepatitis: Insight on hedgehog/Gli and Wnt/β-catenin signaling crosstalk[J]. Biochem Pharmacol, 2020, 177: 113942. DOI: 10.1016/j.bcp.2020.113942. [45] ZHANG MJ, HAUGHEY M, WANG NY, et al. Targeting the Wnt signaling pathway through R-spondin 3 identifies an anti-fibrosis treatment strategy for multiple organs[J]. PLoS One, 2020, 15( 3): e0229445. DOI: 10.1371/journal.pone.0229445. [46] HE WW, YANG RG, LUO TT. Effect of ginsenoside Rg1 on liver fibrosis in mice with non-alcoholic fatty liver disease[J]. J Chin Med Mater, 2021, 44( 5): 1208- 1212. DOI: 10.13863/j.issn1001-4454.2021.05.032.贺微微, 杨仁国, 罗婷婷. 人参皂苷Rg1对非酒精性脂肪性肝病小鼠肝纤维化的作用[J]. 中药材, 2021, 44( 5): 1208- 1212. DOI: 10.13863/j.issn1001-4454.2021.05.032. [47] LI QJ, GONG YQ, WANG Y, et al. Sirt1 promotes the restoration of hepatic progenitor cell(HPC)-mediated liver fatty injury in NAFLD through activating the Wnt/β-catenin signal pathway[J]. Front Nutr, 2021, 8: 791861. DOI: 10.3389/fnut.2021.791861. [48] YU J, ZHAO Y, XU LL, et al. Liraglutide attenuates hepatic oxidative stress, inflammation, and apoptosis in streptozotocin-induced diabetic mice by modulating the Wnt/β-catenin signaling pathway[J]. Mediators Inflamm, 2023, 2023: 8974960. DOI: 10.1155/2023/8974960. [49] XUE C, CHU QF, SHI QM, et al. Wnt signaling pathways in biology and disease: Mechanisms and therapeutic advances[J]. Signal Transduct Target Ther, 2025, 10( 1): 106. DOI: 10.1038/s41392-025-02142-w. [50] ZHENG GG, LIN SQ, WANG SJ, et al. Regulation of natural products on Wnt/β-catenin signaling pathway in diseases[J]. Am J Chin Med, 2025, 53( 3): 709- 735. DOI: 10.1142/S0192415X25500272. [51] DUSPARA K, BOJANIC K, PEJIC JI, et al. Targeting the Wnt signaling pathway in liver fibrosis for drug options: An update[J]. J Clin Transl Hepatol, 2021, 9( 6): 960- 971. DOI: 10.14218/JCTH.2021.00065. -



 PDF下载 ( 1776 KB)
PDF下载 ( 1776 KB)

 下载:
下载:


