铁过载和铁死亡对自身免疫性肝炎发生发展的影响及作用机制
DOI: 10.12449/JCH251128
The impact of iron overload and ferroptosis on the development and progression of autoimmune hepatitis and their mechanism of action
-
摘要: 自身免疫性肝炎(AIH)是一种由免疫功能紊乱引起的炎症性疾病,其致病机制尚待系统解析。近年来,大量研究发现铁稳态失衡和铁死亡与AIH的发病机制及疾病进展密切相关。本文综述了铁过载与铁死亡在AIH中的病理机制及其作用,旨在为AIH的机制研究和临床治疗提供新的启示与理论依据。Abstract: Autoimmune hepatitis (AIH) is an inflammatory disease caused by immune dysfunction, and its pathogenic mechanism remains unclear. In recent years, a large number of studies have shown that iron homeostasis imbalance and ferroptosis are closely associated with the pathogenesis and progression of AIH. This article reviews the pathological mechanism and impact of iron overload and ferroptosis in AIH, in order to provide new insights and theoretical bases for research on the mechanism and clinical treatment of AIH.
-
Key words:
- Hepatitis, Autoimmune /
- Iron Overload /
- Ferroptosis
-
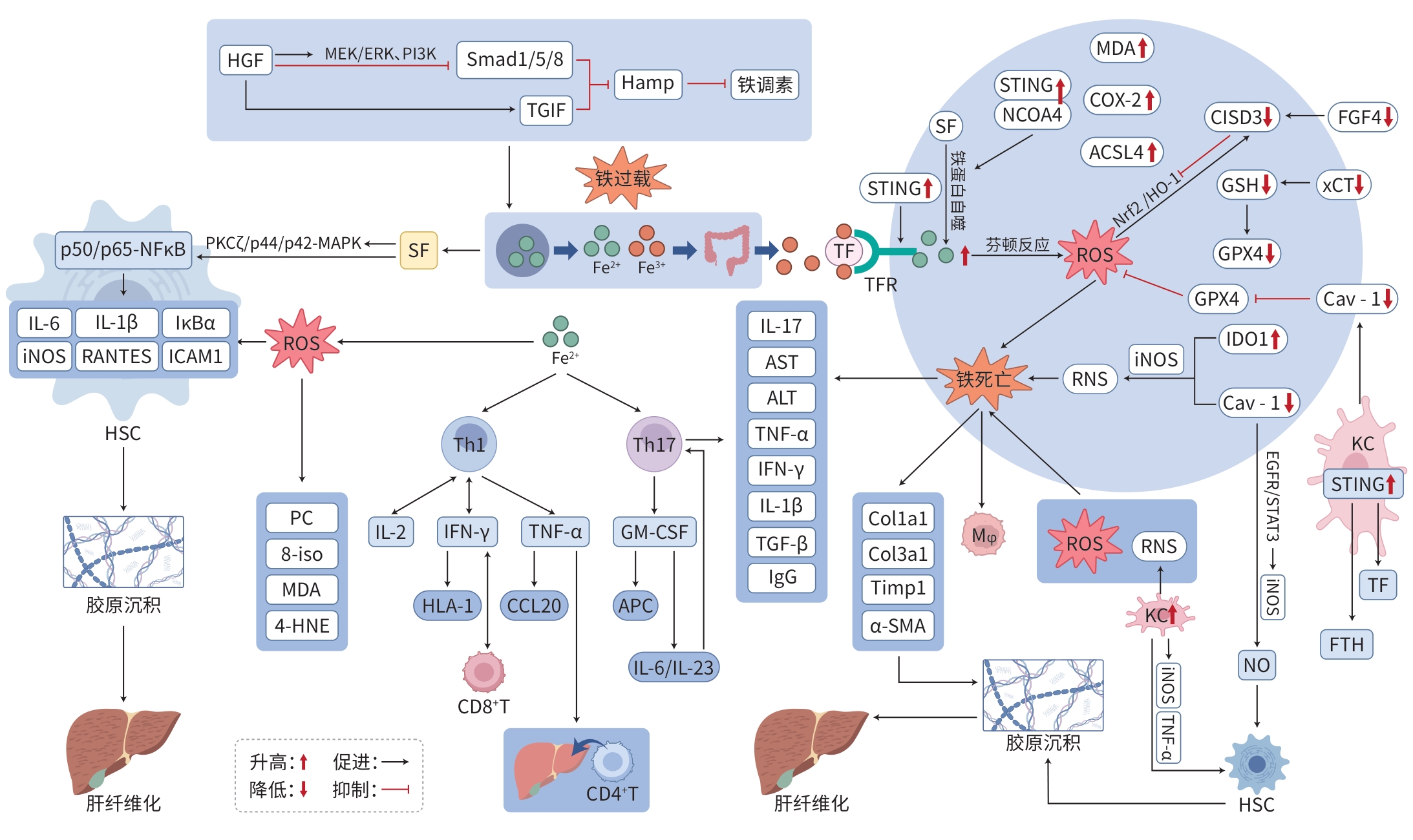
注: MEK/ERK,丝裂原活化蛋白激酶/细胞外信号调节激酶;PI3K,磷脂酰肌醇-3激酶;TGIF,转录共抑制因子;Hamp,铁调素基因;4-HNE,反式-4-羟基-2-壬烯醛定量检测;8-iso,8-异前列腺素;PC,蛋白质羰基化产物;HLA-Ⅰ,人类白细胞抗原Ⅰ类分子;CCL20,趋化因子配体20;APC,抗原呈递细胞;PKCζ,蛋白激酶Cζ;p50/p65-NFκB,核因子κB亚基p50/p65;ICAM1,细胞间黏附分子1;IκBα,核因子κB抑制蛋白α;RANTES,趋化因子配体5;ACSL4,酰基辅酶A长链家族成员4;TF,转铁蛋白;TFR,转铁蛋白受体;p44/p42-MAPK,p44/p42丝裂原活化蛋白激酶;FTH,铁蛋白重链;Mφ,巨噬细胞;Col1a1,Ⅰ型胶原蛋白α1链;Col3a1,Ⅲ型胶原蛋白α1链;Timp1,金属蛋白酶组织抑制因子1;α-SMA,α-平滑肌肌动蛋白;EGFR/STAT3,表皮生长因子受体/信号转导与转录激活因子3;KC,Kupffer细胞。
图 1 铁过载及铁死亡影响AIH的作用机制
Figure 1. Mechanism diagram of the roles of iron overload and ferroptosis in AIH
-
[1] YANG XY, DANG XH, JIANG Y. Research advances in pharmacotherapy for autoimmune hepatitis[J]. J Clin Hepatol, 2023, 39( 4): 915- 921. DOI: 10.3969/j.issn.1001-5256.2023.04.026.杨秀英, 党小红, 姜洋. 自身免疫性肝炎的药物治疗进展[J]. 临床肝胆病杂志, 2023, 39( 4): 915- 921. DOI: 10.3969/j.issn.1001-5256.2023.04.026. [2] MIELI-VERGANI G, VERGANI D, CZAJA AJ, et al. Autoimmune hepatitis[J]. Nat Rev Dis Primers, 2018, 4: 18017. DOI: 10.1038/nrdp.2018.17. [3] MACK CL, ADAMS D, ASSIS DN, et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American Association for the Study of Liver Diseases[J]. Hepatology, 2020, 72( 2): 671- 722. DOI: 10.1002/hep.31065. [4] ZHANG KH, LI JC, SHI Z, et al. Ginsenosides regulates innate immunity to affect immune microenvironment of AIH through hippo-YAP/TAZ signaling pathway[J]. Front Immunol, 2022, 13: 851560. DOI: 10.3389/fimmu.2022.851560. [5] MANCARDI D, MEZZANOTTE M, ARRIGO E, et al. Iron overload, oxidative stress, and ferroptosis in the failing heart and liver[J]. Antioxidants(Basel), 2021, 10( 12): 1864. DOI: 10.3390/antiox10121864. [6] LI YK, HUANG XL, WANG JJ, et al. Regulation of iron homeostasis and related diseases[J]. Mediators Inflamm, 2020, 2020: 6062094. DOI: 10.1155/2020/6062094. [7] SHEN L, WANG XH, ZHAI CL, et al. Ferroptosis: A potential therapeutic target in autoimmune disease(Review)[J]. Exp Ther Med, 2023, 26( 2): 368. DOI: 10.3892/etm.2023.12067. [8] LI JY, FENG YH, LI YX, et al. Ferritinophagy: A novel insight into the double-edged sword in ferritinophagy-ferroptosis axis and human diseases[J]. Cell Prolif, 2024, 57( 7): e13621. DOI: 10.1111/cpr.13621. [9] ANANDHAN A, DODSON M, SHAKYA A, et al. NRF2 controls iron homeostasis and ferroptosis through HERC2 and VAMP8[J]. Sci Adv, 2023, 9( 5): eade9585. DOI: 10.1126/sciadv.ade9585. [10] YAZDALI KOYLU N, KOYLU B, SOKMENSUER C, et al. In the presence of autoantibodies and iron overload, do not judge a book by its cover: A case report[J]. Hepatol Forum, 2021, 2( 2): 76- 79. DOI: 10.14744/hf.2021.2021.0013. [11] CHAUHAN S, ZACKRIA R, MUKHOPADHYAY DK. Autoimmune hepatitis in the setting of iron overload secondary to heterozygous HFE gene mutation[J]. Cureus, 2022, 14( 8): e27614. DOI: 10.7759/cureus.27614. [12] TAUBERT R, HARDTKE-WOLENSKI M, NOYAN F, et al. Hyperferritinemia and hypergammaglobulinemia predict the treatment response to standard therapy in autoimmune hepatitis[J]. PLoS One, 2017, 12( 6): e0179074. DOI: 10.1371/journal.pone.0179074. [13] ZHENG H, YANG F, DENG K, et al. Relationship between iron overload caused by abnormal hepcidin expression and liver disease: A review[J]. Medicine(Baltimore), 2023, 102( 11): e33225. DOI: 10.1097/MD.0000000000033225. [14] LYBEROPOULOU A, CHACHAMI G, GATSELIS NK, et al. Low serum hepcidin in patients with autoimmune liver diseases[J]. PLoS One, 2015, 10( 8): e0135486. DOI: 10.1371/journal.pone.0135486. [15] GOODNOUGH JB, RAMOS E, NEMETH E, et al. Inhibition of hepcidin transcription by growth factors[J]. Hepatology, 2012, 56( 1): 291- 299. DOI: 10.1002/hep.25615. [16] BOVENSIEPEN CS, SCHAKAT M, SEBODE M, et al. TNF-producing Th1 cells are selectively expanded in liver infiltrates of patients with autoimmune hepatitis[J]. J Immunol, 2019, 203( 12): 3148- 3156. DOI: 10.4049/jimmunol.1900124. [17] CHEN HR, HAN ZY, FAN YY, et al. CD4+ T-cell subsets in autoimmune hepatitis: A review[J]. Hepatol Commun, 2023, 7( 10): e0269. DOI: 10.1097/HC9.0000000000000269. [18] ZHAO L, TANG YL, YOU ZR, et al. Interleukin-17 contributes to the pathogenesis of autoimmune hepatitis through inducing hepatic interleukin-6 expression[J]. PLoS One, 2011, 6( 4): e18909. DOI: 10.1371/journal.pone.0018909. [19] WANG ZZ, YIN WJ, ZHU LZ, et al. Iron drives T helper cell pathogenicity by promoting RNA-binding protein PCBP1-mediated proinflammatory cytokine production[J]. Immunity, 2018, 49( 1): 80- 92. DOI: 10.1016/j.immuni.2018.05.008. [20] CZAJA AJ. Review article: Iron disturbances in chronic liver diseases other than haemochromatosis-pathogenic, prognostic, and therapeutic implications[J]. Aliment Pharmacol Ther, 2019, 49( 6): 681- 701. DOI: 10.1111/apt.15173. [21] GAO H, JIN ZM, BANDYOPADHYAY G, et al. Aberrant iron distribution via hepatocyte-stellate cell axis drives liver lipogenesis and fibrosis[J]. Cell Metab, 2022, 34( 8): 1201- 1213. DOI: 10.1016/j.cmet.2022.07.006. [22] KAFFE ET, RIGOPOULOU EI, KOUKOULIS GK, et al. Oxidative stress and antioxidant status in patients with autoimmune liver diseases[J]. Redox Rep, 2015, 20( 1): 33- 41. DOI: 10.1179/1351000214Y.0000000101. [23] CHEN Y, LEI Y, WANG H, et al. Sophoricoside attenuates autoimmune-mediated liver injury through the regulation of oxidative stress and the NF-κB signaling pathway[J]. Int J Mol Med, 2023, 52( 3): 78. DOI: 10.3892/ijmm.2023.5281. [24] ZHANG Y, ZHANG YY, XIE Y, et al. Multitargeted inhibition of hepatic fibrosis in chronic iron-overloaded mice by Salvia miltiorrhiza[J]. J Ethnopharmacol, 2013, 148( 2): 671- 681. DOI: 10.1016/j.jep.2013.05.028. [25] GARCIA-CASAL MN, PASRICHA SR, MARTINEZ RX, et al. Serum or plasma ferritin concentration as an index of iron deficiency and overload[J]. Cochrane Database Syst Rev, 2021, 5( 5): CD011817. DOI: 10.1002/14651858.CD011817.pub2. [26] CHEN QL, GAO M, YANG H, et al. Serum ferritin levels are associated with advanced liver fibrosis in treatment-naive autoimmune hepatitis[J]. BMC Gastroenterol, 2022, 22( 1): 23. DOI: 10.1186/s12876-022-02098-z. [27] SUNGKAR T, ROZI MF, DAIRI LB, et al. Serum ferritin levels: A potential biomarker to represent Child-Turcotte-Pugh score among decompensated liver cirrhosis patients[J]. Malays J Med Sci, 2019, 26( 2): 59- 65. DOI: 10.21315/mjms2019.26.2.7. [28] RUDDELL RG, HOANG-LE D, BARWOOD JM, et al. Ferritin functions as a proinflammatory cytokine via iron-independent PKC-ζ/NFκB-regulated signalling in rat hepatic stellate cells[J]. Hepatology, 2009, 49( 3): 887- 900. DOI: 10.1002/hep.22716. [29] WU AM, FENG B, YU J, et al. Fibroblast growth factor 21 attenuates iron overload-induced liver injury and fibrosis by inhibiting ferroptosis[J]. Redox Biol, 2021, 46: 102131. DOI: 10.1016/j.redox.2021.102131. [30] LIU Y, CHEN H, HAO JH, et al. Characterization and functional prediction of the microRNAs differentially expressed in a mouse model of concanavalin A-induced autoimmune hepatitis[J]. Int J Med Sci, 2020, 17( 15): 2312- 2327. DOI: 10.7150/ijms.47766. [31] DEVISSCHER L, van COILLIE S, HOFMANS S, et al. Discovery of novel, drug-like ferroptosis inhibitors with in vivo efficacy[J]. J Med Chem, 2018, 61( 22): 10126- 10140. DOI: 10.1021/acs.jmedchem.8b01299. [32] DENG GH, LI YJ, MA SY, et al. Caveolin-1 dictates ferroptosis in the execution of acute immune-mediated hepatic damage by attenuating nitrogen stress[J]. Free Radic Biol Med, 2020, 148: 151- 161. DOI: 10.1016/j.freeradbiomed.2019.12.026. [33] JIANG HM, FANG Y, WANG YX, et al. FGF4 improves hepatocytes ferroptosis in autoimmune hepatitis mice via activation of CISD3[J]. Int Immunopharmacol, 2023, 116: 109762. DOI: 10.1016/j.intimp.2023.109762. [34] ZHU LJ, CHEN DZ, ZHU Y, et al. GPX4-regulated ferroptosis mediates S100-induced experimental autoimmune hepatitis associated with the Nrf2/HO-1 signaling pathway[J]. Oxid Med Cell Longev, 2021, 2021: 6551069. DOI: 10.1155/2021/6551069. [35] ZENG T, DENG GH, ZHONG WC, et al. Indoleamine 2, 3-dioxygenase 1 enhances hepatocytes ferroptosis in acute immune hepatitis associated with excess nitrative stress[J]. Free Radic Biol Med, 2020, 152: 668- 679. DOI: 10.1016/j.freeradbiomed.2020.01.009. [36] DECOUT A, KATZ JD, VENKATRAMAN S, et al. The cGAS-STING pathway as a therapeutic target in inflammatory diseases[J]. Nat Rev Immunol, 2021, 21( 9): 548- 569. DOI: 10.1038/s41577-021-00524-z. [37] WU J, LIU QJ, ZHANG XF, et al. The interaction between STING and NCOA4 exacerbates lethal sepsis by orchestrating ferroptosis and inflammatory responses in macrophages[J]. Cell Death Dis, 2022, 13( 7): 653. DOI: 10.1038/s41419-022-05115-x. [38] ZHAO JM, YI ZY, DENG GH, et al. STING modulates iron metabolism to promote liver injury and inflammation in acute immune hepatitis[J]. Free Radic Biol Med, 2024, 210: 367- 377. DOI: 10.1016/j.freeradbiomed.2023.11.038. [39] YAN B, AI YW, SUN Q, et al. Membrane damage during ferroptosis is caused by oxidation of phospholipids catalyzed by the oxidoreductases POR and CYB5R1[J]. Mol Cell, 2021, 81( 2): 355- 369. DOI: 10.1016/j.molcel.2020.11.024. [40] KHALEEL A, EL-SHEAKH AR, SUDDEK GM. Celecoxib abrogates concanavalin A-induced hepatitis in mice: Possible involvement of Nrf2/HO-1, JNK signaling pathways and COX-2 expression[J]. Int Immunopharmacol, 2023, 121: 110442. DOI: 10.1016/j.intimp.2023.110442. [41] HOU YW, CHEN C, LI ZR, et al. Protective effect of quercetin against macrophage-mediated hepatocyte injury via anti-inflammation, anti-apoptosis and inhibition of ferroptosis[J]. Autoimmunity, 2024, 57( 1): 2350202. DOI: 10.1080/08916934.2024.2350202. [42] LI ZR, CHEN C, GUO D, et al. Role and mechanism of action of Yinchenhao Decoction in inhibiting ferroptosis of hepatocytes in mice with autoimmune hepatitis[J]. J Clin Hepatol, 2024, 40( 3): 502- 508. DOI: 10.12449/JCH240311.李竹蓉, 陈晨, 郭地, 等. 茵陈蒿汤对自身免疫性肝炎小鼠肝细胞铁死亡的抑制作用及其机制分析[J]. 临床肝胆病杂志, 2024, 40( 3): 502- 508. DOI: 10.12449/JCH240311. [43] YANG Y, WANG Y, GUO L, et al. Interaction between macrophages and ferroptosis[J]. Cell Death Dis, 2022, 13( 4): 355. DOI: 10.1038/s41419-022-04775-z. [44] YU YY, JIANG L, WANG H, et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis[J]. Blood, 2020, 136( 6): 726- 739. DOI: 10.1182/blood.2019002907. [45] SU WT, GAO WC, ZHANG R, et al. TAK1 deficiency promotes liver injury and tumorigenesis via ferroptosis and macrophage cGAS-STING signalling[J]. JHEP Rep, 2023, 5( 5): 100695. DOI: 10.1016/j.jhepr.2023.100695. [46] FERNANDEZ-ROJO MA, RAMM GA. Caveolin-1 function in liver physiology and disease[J]. Trends Mol Med, 2016, 22( 10): 889- 904. DOI: 10.1016/j.molmed.2016.08.007. [47] BEYAZIT Y, EFE C, TANOGLU A, et al. Nitric oxide is a potential mediator of hepatic inflammation and fibrogenesis in autoimmune hepatitis[J]. Scand J Gastroenterol, 2015, 50( 2): 204- 210. DOI: 10.3109/00365521.2014.974203. [48] SEO HY, LEE SH, HAN E, et al. Evogliptin directly inhibits inflammatory and fibrotic signaling in isolated liver cells[J]. Int J Mol Sci, 2022, 23( 19): 11636. DOI: 10.3390/ijms231911636. -



 PDF下载 ( 1705 KB)
PDF下载 ( 1705 KB)

 下载:
下载:


