中医药联合免疫检查点抑制剂对中晚期肝细胞癌的治疗作用与机制
DOI: 10.12449/JCH251130
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:李永琪、李妍秋负责论文选题,框架设计,撰写论文;孙丽娜、王超冉负责资料收集,修订论文;冯颖负责指导撰写文章、论文审阅与修订;王宪波负责拟定写作思路并最后定稿。
Efficacy and mechanism of traditional Chinese medicine combined with immune checkpoint inhibitor in treatment of advanced hepatocellular carcinoma
-
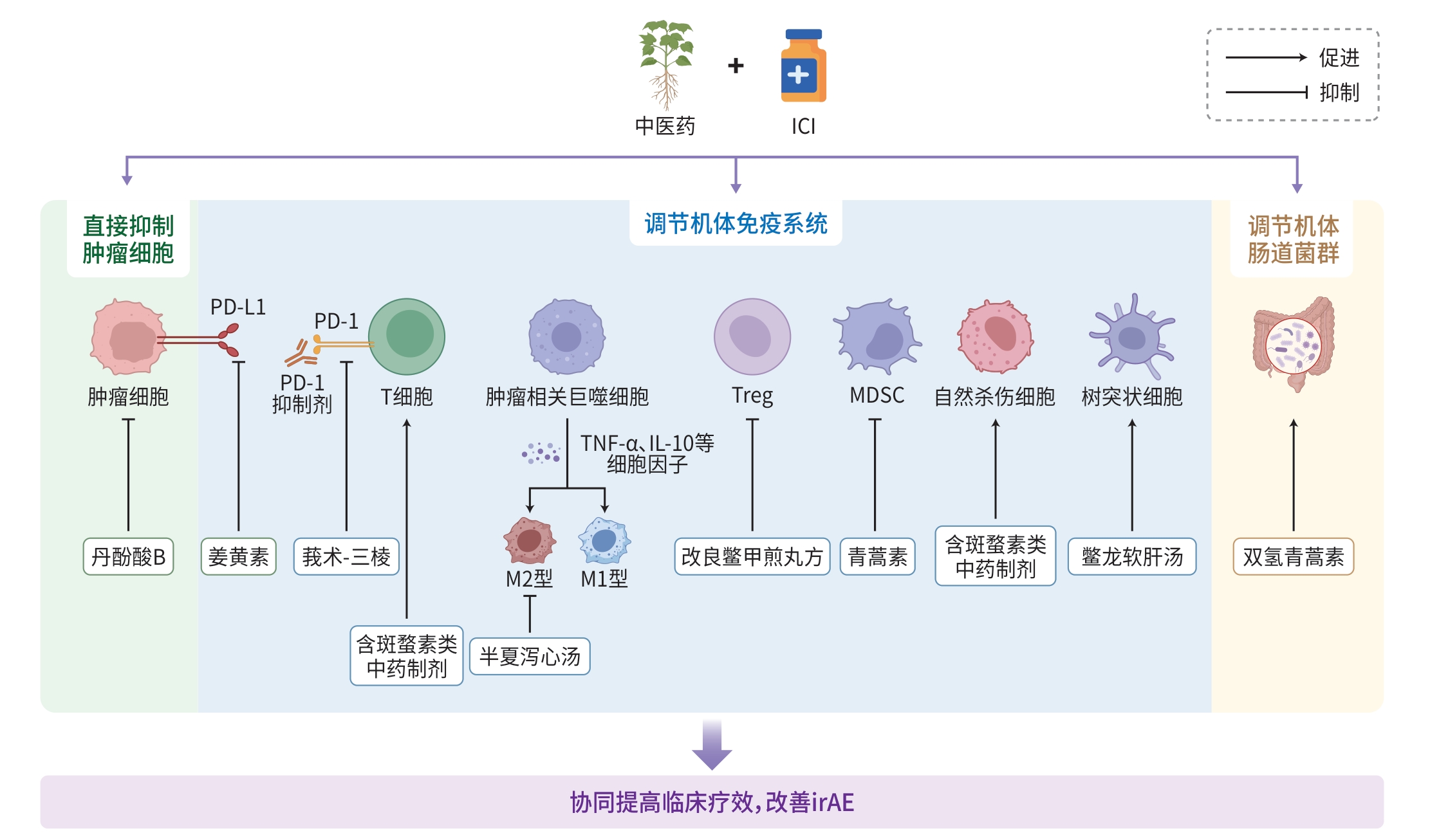
摘要: 肝细胞癌(HCC)是我国发病率和死亡率均较高的恶性肿瘤,其中晚期阶段是当前治疗中的难点。免疫检查点抑制剂(ICI)作为新兴治疗方案在临床中虽显示出一定的效果,但存在总体应答率低和免疫相关不良事件发生较多等问题。中医药在中晚期HCC治疗中具有独特优势。本文通过系统回顾近年相关研究发现,中医药联合ICI可通过多成分、多靶点、多途径协同机制,在中晚期HCC治疗中发挥控制疾病进展、延长患者生存时间及减少免疫相关不良事件等作用,其潜在机制可能涉及直接抑制肿瘤细胞、调节机体免疫系统及肠道菌群等多个方面。Abstract: Hepatocellular carcinoma (HCC) is a malignant tumor with high incidence and mortality rates in China, and advanced HCC is a difficult issue in current treatment. Immune checkpoint inhibitor (ICI), as an emerging treatment regimen, has shown a certain effect in clinical practice, but there are still problems such as low overall response rate and a high incidence rate of immune-related adverse events (irAEs). Traditional Chinese medicine exhibits unique advantages in the treatment of advanced HCC. Through a retrospective analysis of related studies in recent years, this article shows that traditional Chinese medicine combined with ICI can control disease progression, prolong survival time, and reduce irAEs in the treatment of advanced HCC through the synergistic effect between multiple components, targets, and pathways. The potential mechanism of this treatment modality may involve various aspects such as the direct inhibition of tumor cells and the regulation of immune system and intestinal flora.
-
[1] ZHENG RS, CHEN R, HAN BF, et al. Analysis on the prevalence of malignant tumors in China in 2022[J]. Chin J Oncol, 2024, 46( 3): 221- 231. DOI: 10.3760/cma.j.cn112152-20240119-00035.郑荣寿, 陈茹, 韩冰峰, 等. 2022年中国恶性肿瘤流行情况分析[J]. 中华肿瘤杂志, 2024, 46( 3): 221- 231. DOI: 10.3760/cma.j.cn112152-20240119-00035. [2] PARK JW, CHEN MS, COLOMBO M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: The BRIDGE Study[J]. Liver Int, 2015, 35( 9): 2155- 2166. DOI: 10.1111/liv.12818. [3] KWON MJ, CHANG S, KIM JH, et al. Factors associated with the survival outcomes of patients with untreated hepatocellular carcinoma: An analysis of nationwide data[J]. Front Oncol, 2023, 13: 1142661. DOI: 10.3389/fonc.2023.1142661. [4] ZHU LZ, XU XL, ABUDUSALAMU AN, et al. Research advances in immunotherapy for hepatocellular carcinoma[J]. J Clin Hepatol, 2023, 39( 5): 1197- 1203. DOI: 10.3969/j.issn.1001-5256.2023.05.031.朱丽珍, 许晓磊, 阿卜杜萨拉木·艾尼, 等. 肝细胞癌免疫治疗研究进展[J]. 临床肝胆病杂志, 2023, 39( 5): 1197- 1203. DOI: 10.3969/j.issn.1001-5256.2023.05.031. [5] RULI TM Jr, POLLACK ED, LODH A, et al. Immune checkpoint inhibitors in hepatocellular carcinoma and their hepatic-related side effects: A review[J]. Cancers, 2024, 16( 11): 2042. DOI: 10.3390/cancers16112042. [6] YU YX, WANG S, LIU ZN, et al. Traditional Chinese medicine in the era of immune checkpoint inhibitor: Theory, development, and future directions[J]. Chin Med, 2023, 18( 1): 59. DOI: 10.1186/s13020-023-00751-7. [7] YOUNIS A, GRIBBEN J. Immune checkpoint inhibitors: Fundamental mechanisms, current status and future directions[J]. Immuno, 2024, 4( 3): 186- 210. DOI: 10.3390/immuno4030013. [8] EL-KHOUEIRY AB, SANGRO B, YAU T, et al. Nivolumab in patients with advanced hepatocellular carcinoma(CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial[J]. Lancet, 2017, 389( 10088): 2492- 2502. DOI: 10.1016/S0140-6736(17)31046-2. [9] FINN RS, QIN SK, IKEDA M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma[J]. N Engl J Med, 2020, 382( 20): 1894- 1905. DOI: 10.1056/NEJMoa1915745. [10] SPRANGER S, DAI D, HORTON B, et al. Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy[J]. Cancer Cell, 2017, 31( 5): 711- 723. e 4. DOI: 10.1016/j.ccell.2017.04.003. [11] POSTOW MA, SIDLOW R, HELLMANN MD. Immune-related adverse events associated with immune checkpoint blockade[J]. N Engl J Med, 2018, 378( 2): 158- 168. DOI: 10.1056/NEJMra1703481. [12] MENG XF, WANG XB. Clinical thinking and application of spleen-strengthening liver-protecting and nude eliminating methods for liver cancer used by WANG Xian-bo[J]. Beijing J Tradit Chin Med, 2019, 38( 7): 654- 657. DOI: 10.16025/j.1674-1307.2019.07.007.孟晓峰, 王宪波. 王宪波健脾护肝散结法治疗肝癌的临证思路与临床应用[J]. 北京中医药, 2019, 38( 7): 654- 657. DOI: 10.16025/j.1674-1307.2019.07.007. [13] LIU FX, HAN Y, SUN T, et al. Clinical efficacy of a self-formulated Jiedu Shugan Huoxue formula combined with PD-1 inhibitors in treating primary liver cancer after microwave ablation[J]. Acta Chin Med Pharmacol, 2024, 52( 12): 93- 98. DOI: 10.19664/j.cnki.1002-2392.240256.刘凤新, 韩钰, 孙韬, 等. 自拟解毒疏肝活血方联合PD-1抑制剂治疗原发性肝癌微波消融术后的临床效果[J]. 中医药学报, 2024, 52( 12): 93- 98. DOI: 10.19664/j.cnki.1002-2392.240256. [14] ZHANG LJ. Efficacy and safety of hepatoma Ⅰ formula plus minus plus and minus plus PD-1 inhibitor in the treatment of advanced primary hepatoma[D]. Lanzhou: Gansu University of Chinese Medicine, 2023.张丽娟. 肝癌Ⅰ号方加减联合PD-1抑制剂治疗中晚期原发性肝癌的疗效及安全性[D]. 兰州: 甘肃中医药大学, 2023. [15] ZHOU K, YUAN W, WU J, et al. Clinical efficacy of Shugan Jianpi Jiedu decoction combined with tirelizumab and bevacizumab on patients with advanced liver cancer[J]. Chin Tradit Pat Med, 2024, 46( 12): 4221- 4224. DOI: 10.3969/j.issn.1001-1528.2024.12.052.周坤, 袁维, 伍静, 等. 疏肝健脾解毒方联合替雷利珠单抗及贝伐珠单抗对晚期肝癌患者的临床疗效[J]. 中成药, 2024, 46( 12): 4221- 4224. DOI: 10.3969/j.issn.1001-1528.2024.12.052. [16] ZHAI FY, XU YQ. Advances in the study of traditional Chinese medicine to prevention and treatment immune-related adverse reactions of PD-1/PD-L1inhibitors[J]. Clin J Tradit Chin Med, 2022, 34( 9): 1773- 1777. DOI: 10.16448/j.cjtcm.2022.0945.翟凡叶, 许尤琪. 中医药防治PD-1/PD-L1抑制剂免疫相关不良反应的研究进展[J]. 中医药临床杂志, 2022, 34( 9): 1773- 1777. DOI: 10.16448/j.cjtcm.2022.0945. [17] YU D, HE SL, SHEN J, et al. Clinical observation of Ruyi Jinhuang powder combined with ICIs in the treatment of advanced liver cancer complicated with dampness and heat syndrome of liver and gallbladder[J]. China Pharm, 2023, 34( 12): 1488- 1492. DOI: 10.6039/j.issn.1001-0408.2023.12.15.喻丹, 何胜利, 沈婕, 等. 如意金黄散联合ICIs治疗肝癌晚期伴肝胆湿热证者的临床观察[J]. 中国药房, 2023, 34( 12): 1488- 1492. DOI: 10.6039/j.issn.1001-0408.2023.12.15. [18] WANG Y. Clinical observation on the therapeutic effect of SiWuXiaoFeng decoction on immune checkpoint inhibitor-associated skin toxicity[D]. Changchun: Changchun University of Chinese Medicine, 2024.王燕. 四物消风饮治疗免疫检查点抑制剂相关皮肤毒性的临床疗效观察[D]. 长春: 长春中医药大学, 2024. [19] CHEN HZ, LIANG WL, GU Z, et al. Etiology and pathogeny analysis and treatment exploration in traditional Chinese medicine of immune-related adverse reactions of PD-1/PD-L1 inhibitors[J]. World Chin Med, 2021, 16( 9): 1386- 1390, 1399. DOI: 10.3969/j.issn.1673-7202.2021.09.007.陈泓志, 梁伟林, 顾瞻, 等. 程序性细胞死亡蛋白-1及其配体抑制剂免疫相关不良反应的中医病因病机及治法[J]. 世界中医药, 2021, 16( 9): 1386- 1390, 1399. DOI: 10.3969/j.issn.1673-7202.2021.09.007. [20] QIU WL, SUN QQ, QIU B. Action mechanism progress of Chinese medicinal and its active ingredients in treatment of hepatic carcinoma[J]. Inf Tradit Chin Med, 2022, 39( 5): 79- 84. DOI: 10.19656/j.cnki.1002-2406.20220515.邱文亮, 孙倩倩, 邱冰. 中药及其活性成分在肝癌治疗中的作用机制研究进展[J]. 中医药信息, 2022, 39( 5): 79- 84. DOI: 10.19656/j.cnki.1002-2406.20220515. [21] CHENG QL, LIU L, BAI CC, et al. Mechanism of action of five classic prescriptions in treatment of hepatocellular carcinoma based on network pharmacology[J]. J Clin Hepatol, 2021, 37( 8): 1848- 1855. DOI: 10.3969/j.issn.1001-5256.2021.08.020.程秋骆, 刘柳, 白长川, 等. 基于网络药理学探析5种经典方剂治疗肝细胞癌的作用机制[J]. 临床肝胆病杂志, 2021, 37( 8): 1848- 1855. DOI: 10.3969/j.issn.1001-5256.2021.08.020. [22] QIAO XP, LUO C, DING XS, et al. Research progress on active components and anticancer molecular mechanism of Huaier[J]. Chem Life, 2023, 43( 1): 124- 129. DOI: 10.13488/j.smhx.20220772.乔旭鹏, 罗程, 丁煦审, 等. 槐耳活性成分及抗癌分子机制[J]. 生命的化学, 2023, 43( 1): 124- 129. DOI: 10.13488/j.smhx.20220772. [23] LIU HY, LI X, ZHANG CW, et al. GJB2 promotes HCC progression by activating glycolysis through cytoplasmic translocation and generating a suppressive tumor microenvironment based on single cell RNA sequencing[J]. Adv Sci, 2024, 11( 39): 2402115. DOI: 10.1002/advs.202402115. [24] GUO HL, LI J, ZHANG Q, et al. Mechanism of rhizoma curcumae-rhizoma sparganii against hepatocellular carcinoma based on PD-1 mediated NF-κB pathway[J]. J Mod Oncol, 2024, 32( 7): 1179- 1185. DOI: 10.3969/j.issn.1672-4992.2024.07.001.郭洪麟, 李静, 张琦, 等. 基于PD-1介导NF-κB通路探讨莪术-三棱抗肝癌的机制[J]. 现代肿瘤医学, 2024, 32( 7): 1179- 1185. DOI: 10.3969/j.issn.1672-4992.2024.07.001. [25] GUO L, LI HB, FAN TL, et al. Synergistic efficacy of curcumin and anti-programmed cell death-1 in hepatocellular carcinoma[J]. Life Sci, 2021, 279: 119359. DOI: 10.1016/j.lfs.2021.119359. [26] ZHANG M, WANG L, LIU W, et al. Targeting inhibition of accumulation and function of myeloid-derived suppressor cells by artemisinin via PI3K/AKT, mTOR, and mapk pathways enhances anti-PD-L1 immunotherapy in melanoma and liver tumors[J]. J Immunol Res, 2022, 2022: 2253436. DOI: 10.1155/2022/2253436. [27] TIAN XC, LIU F, WANG ZJ, et al. Modified Biejia Jianwan decoction restrains PD-L1-mediated immune evasion through the HIF-1α/STAT3/NF-κB signaling pathway[J]. J Ethnopharmacol, 2024, 322: 117577. DOI: 10.1016/j.jep.2023.117577. [28] HE L, LIU XP, CHEN GS, et al. Banxia Xiexin decoction inhibits macrophage polarization to M2Phenotype to sensitize hepatocellular carcinoma to PD-1 monoclonal antibody[J]. Pharmacol Clin Chin Mater Med, 2025, 41( 4): 21- 28. DOI: 10.13412/j.cnki.zyyl.20241115.003.何玲, 刘喜平, 陈光顺, 等. 半夏泻心汤抑制巨噬细胞M2型极化增敏PD-1单抗治疗肝细胞癌[J]. 中药药理与临床, 2025, 41( 4): 21- 28. DOI: 10.13412/j.cnki.zyyl.20241115.003. [29] DING H, WANG J, WANG ZG, et al. Clinical effectiveness of cantharidin-containing Chinese medicine preparations on immune function in patients with hepatocellular cancer: A meta-analysis[J]. J Oncol Chin Med, 2020, 2( 1): 85- 91. DOI: 10.19811/j.cnki.ISSN2096-6628.2020.01.020.丁皓, 王婧, 王志刚, 等. 含斑蝥素中药制剂对肝癌患者免疫功能影响的Meta分析[J]. 中医肿瘤学杂志, 2020, 2( 1): 85- 91. DOI: 10.19811/j.cnki.ISSN2096-6628.2020.01.020. [30] WU YN, LI X, ZHANG D, et al. Influence of BeilongRuangan decoction on perliferation of peripheral blood dendritic cells from HBV related hepatocellular carcinoma patients[J]. Liaoning J Tradit Chin Med, 2019, 46( 3): 646- 649, 673. DOI: 10.13192/j.issn.1000-1719.2019.03.060.伍玉南, 李秀, 张冬, 等. 鳖龙软肝汤含药血浆对HBV相关性肝癌患者外周血树突状细胞增殖的影响[J]. 辽宁中医杂志, 2019, 46( 3): 646- 649, 673. DOI: 10.13192/j.issn.1000-1719.2019.03.060. [31] LI HW. Efficacy and mechanism of Huaier combined with immune checkpoint inhibitors against hepatocellular carcinoma[J]. Chengdu: Chengdu University of Traditional Chinese Medicine, 2023.李华伟. 槐耳联合免疫检查点抑制剂治疗肝癌的疗效及机制研究[D]. 成都: 成都中医药大学, 2023. [32] CAO C, LIU ZY, ZHANG X, et al. A clinical study on the effect of Wenyang fuzheng formula combined with PD-1 immunotherapy on the microenvironment of Yang deficiency type of liver cancer[J]. Labeled Immunoass Clin Med, 2024, 31( 6): 1042- 1048. DOI: 10.11748/bjmy.issn.1006-1703.2024.06.012.曹晨, 刘志勇, 张翔, 等. 温阳扶正方联合PD-1免疫治疗影响阳虚型肝癌微环境的临床研究[J]. 标记免疫分析与临床, 2024, 31( 6): 1042- 1048. DOI: 10.11748/bjmy.issn.1006-1703.2024.06.012. [33] BAGLIERI J, BRENNER DA, KISSELEVA T. The role of fibrosis and liver-associated fibroblasts in the pathogenesis of hepatocellular carcinoma[J]. Int J Mol Sci, 2019, 20( 7): 1723. DOI: 10.3390/ijms20071723. [34] AMERATUNGA M, CHÉNARD-POIRIER M, MORENO CANDILEJO I, et al. Neutrophil-lymphocyte ratio kinetics in patients with advanced solid tumours on phase I trials of PD-1/PD-L1 inhibitors[J]. Eur J Cancer, 2018, 89: 56- 63. DOI: 10.1016/j.ejca.2017.11.012. [35] LIU MK, JI MM, CHENG L, et al. Research progress in anti-tumor effect and mechanism of baicalin[J]. J Shanghai Jiao Tong Univ Med Sci, 2021, 41( 2): 246- 250. DOI: 10.3969/j.issn.1674-8115.2021.02.019.刘梦珂, 纪濛濛, 程林, 等. 黄芩苷抗肿瘤作用机制的研究进展[J]. 上海交通大学学报(医学版), 2021, 41( 2): 246- 250. DOI: 10.3969/j.issn.1674-8115.2021.02.019. [36] TIAN LJ, SANG YJ, SUN YJ, et al. The predictive value of systemic immune-inflammation index for immune checkpoint inhibitor treatment-related adverse reactions in patients with primary liver cancer[J]. J Shandong Univ Health Sci, 2024, 62( 6): 48- 53, 75. DOI: 10.6040/j.issn.1671-7554.0.2024.0267.田丽君, 桑玉洁, 孙瑜婧, 等. 全身免疫炎症指数对原发性肝癌患者免疫检查点抑制剂治疗相关不良反应的预测价值[J]. 山东大学学报(医学版), 2024, 62( 6): 48- 53, 75. DOI: 10.6040/j.issn.1671-7554.0.2024.0267. [37] YANG QQ, WANG B, ZHENG QH, et al. A review of gut microbiota-derived metabolites in tumor progression and cancer therapy[J]. Adv Sci, 2023, 10( 15): 2207366. DOI: 10.1002/advs.202207366. [38] LEE SH, CHO SY, YOON Y, et al. Bifidobacterium bifidum strains synergize with immune checkpoint inhibitors to reduce tumour burden in mice[J]. Nat Microbiol, 2021, 6( 3): 277- 288. DOI: 10.1038/s41564-020-00831-6. [39] ZHANG ZQ, SHI XL, JI JM, et al. Dihydroartemisinin increased the abundance of Akkermansia muciniphila by YAP1 depression that sensitizes hepatocellular carcinoma to anti-PD-1 immunotherapy[J]. Front Med, 2023, 17( 4): 729- 746. DOI: 10.1007/s11684-022-0978-2. -



 PDF下载 ( 1058 KB)
PDF下载 ( 1058 KB)

 下载:
下载:


