中性粒细胞外陷阱在肝细胞癌中的作用
DOI: 10.12449/JCH251132
-
摘要: 肝细胞癌(HCC)是全球范围内发病率和死亡率均较高的恶性肿瘤。近年研究表明,中性粒细胞外陷阱(NET)在HCC的发生、发展及免疫逃逸中发挥重要作用。NET由中性粒细胞释放,主要由DNA、组蛋白及抗菌分子组成,除参与免疫防御外,还与HCC的起始、转移和血栓形成密切相关。本文阐述了NET的形成与调控机制,探讨其在HCC起始、转移、免疫逃逸及血栓形成中的潜在机制,同时讨论NET作为HCC诊断与治疗靶点的前景,旨在为未来HCC的精准治疗提供新思路,推动其早期诊断和有效治疗。Abstract: Hepatocellular carcinoma (HCC) is a malignant tumor with high incidence and mortality rates worldwide. Recent studies have shown that neutrophil extracellular traps (NETs) play an important role in the development, progression, and immune escape of HCC. NETs are released by neutrophils and mainly consist of DNA, histones, and antimicrobial molecules, and in addition to immune defense, they are also involved in the initiation, metastasis, and thrombosis of HCC. This article elaborates on the formation and regulatory mechanisms of NETs, explores their potential mechanisms in the initiation, metastasis, immune escape, and thrombosis of HCC, and discusses the prospect of NETs as a target for the diagnosis and treatment of HCC, in order to provide new ideas for the precise treatment of HCC in the future and promote the early diagnosis and effective treatment of HCC.
-
Key words:
- Carcinoma,Hepatocellular /
- Neutrophil Extratrapping /
- Pathologic Processes
-
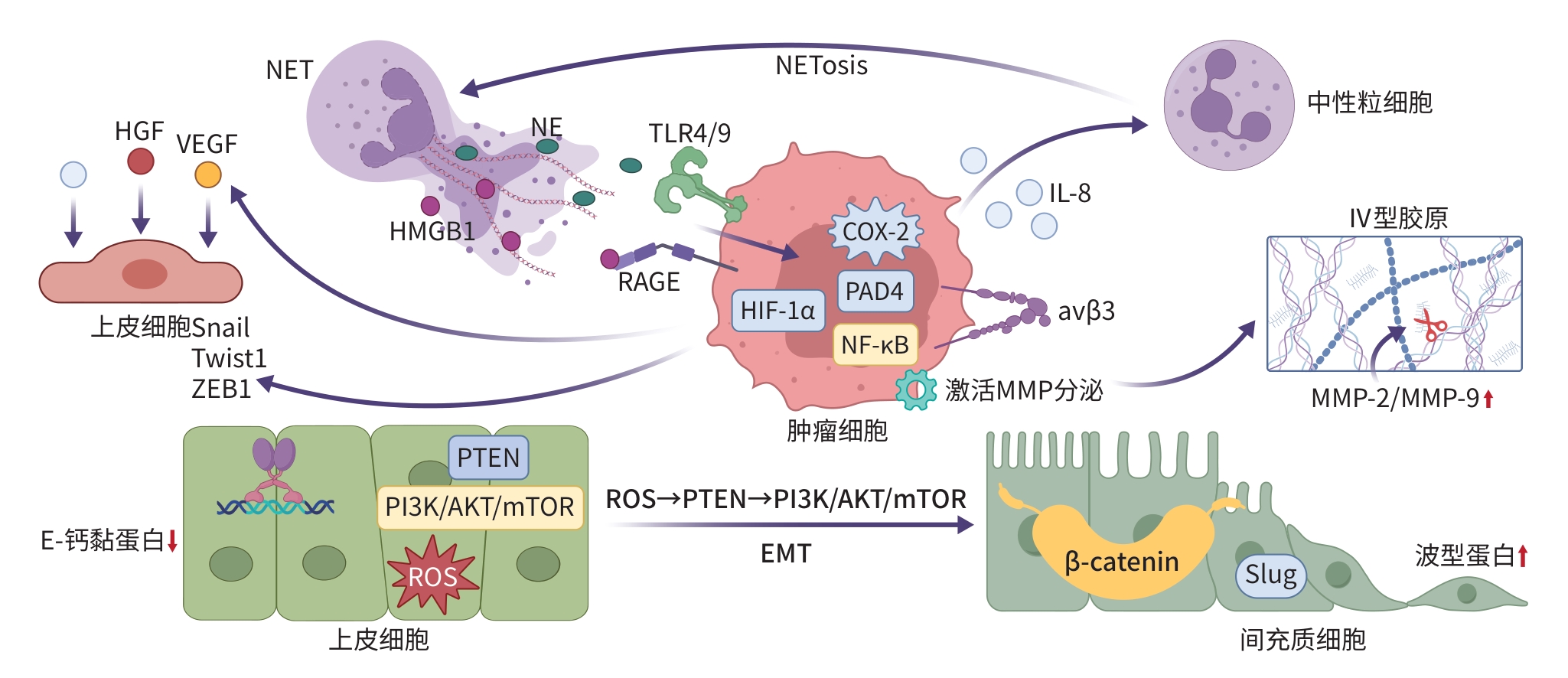
注: NETosis,中性粒细胞胞外陷阱形成;HIF-1α,缺氧诱导因子1α;αvβ3,整合素αvβ3;HGF,肝细胞生长因子; Snail,Snail家族转录因子;Twist1,扭曲因子1;ZEB1,锌指E盒结合同源框蛋白1;PTEN,磷酸酶与张力蛋白同源物;PI3K,磷脂酰肌醇3-激酶;AKT,蛋白激酶B;mTOR,哺乳动物雷帕霉素靶蛋白; β-catenin,β-连环蛋白;Slug,Slug转录因子。
图 1 NET促进HCC转移机制图
Figure 1. Mechanism of neutrophil extracellular traps promoting metastasis in hepatocellular carcinoma
-
[1] LLOVET JM, PINYOL R, YARCHOAN M, et al. Adjuvant and neoadjuvant immunotherapies in hepatocellular carcinoma[J]. Nat Rev Clin Oncol, 2024, 21( 4): 294- 311. DOI: 10.1038/s41571-024-00868-0. [2] VOGEL A, MEYER T, SAPISOCHIN G, et al. Hepatocellular carcinoma[J]. Lancet, 2022, 400( 10360): 1345- 1362. DOI: 10.1016/S0140-6736(22)01200-4. [3] LUO XT, HE X, ZHANG XM, et al. Hepatocellular carcinoma: Signaling pathways, targeted therapy, and immunotherapy[J]. MedComm, 2024, 5( 2): e474. DOI: 10.1002/mco2.474. [4] HERRE M, CEDERVALL J, MACKMAN N, et al. Neutrophil extracellular traps in the pathology of cancer and other inflammatory diseases[J]. Physiol Rev, 2023, 103( 1): 277- 312. DOI: 10.1152/physrev.00062.2021. [5] YANG SX, JIA JC, WANG FQ, et al. Targeting neutrophils: Mechanism and advances in cancer therapy[J]. Clin Transl Med, 2024, 14( 3): e1599. DOI: 10.1002/ctm2.1599. [6] HILSCHER MB, SHAH VH. Neutrophil extracellular traps and liver disease[J]. Semin Liver Dis, 2020, 40( 2): 171- 179. DOI: 10.1055/s-0039-3399562. [7] BRINKMANN V, REICHARD U, GOOSMANN C, et al. Neutrophil extracellular traps kill bacteria[J]. Science, 2004, 303( 5663): 1532- 1535. DOI: 10.1126/science.1092385. [8] WANG YJ, DU CJ, ZHANG Y, et al. Composition and function of neutrophil extracellular traps[J]. Biomolecules, 2024, 14( 4): 416. DOI: 10.3390/biom14040416. [9] PAPAYANNOPOULOS V, METZLER KD, HAKKIM A, et al. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps[J]. J Cell Biol, 2010, 191( 3): 677- 691. DOI: 10.1083/jcb.201006052. [10] LI PX, LI M, LINDBERG MR, et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps[J]. J Exp Med, 2010, 207( 9): 1853- 1862. DOI: 10.1084/jem.20100239. [11] METZLER KD, FUCHS TA, NAUSEEF WM, et al. Myeloperoxidase is required for neutrophil extracellular trap formation: Implications for innate immunity[J]. Blood, 2011, 117( 3): 953- 959. DOI: 10.1182/blood-2010-06-290171. [12] SOLLBERGER G, CHOIDAS A, BURN GL, et al. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps[J]. Sci Immunol, 2018, 3( 26): eaar6689. DOI: 10.1126/sciimmunol.aar6689. [13] KENNY EF, HERZIG A, KRÜGER R, et al. Diverse stimuli engage different neutrophil extracellular trap pathways[J]. eLife, 2017, 6: e24437. DOI: 10.7554/eLife.24437. [14] COOLS-LARTIGUE J, SPICER J, MCDONALD B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis[J]. J Clin Invest, 2013, 123( 8): 3446- 3458. DOI: 10.1172/JCI67484. [15] HUCKE F, EMMER H, EMMER R, et al. Changes in the epidemiology of hepatocellular carcinoma in Carinthia, Austria, 2012-2023[J]. Cancers, 2023, 15( 21): 5215. DOI: 10.3390/cancers15215215. [16] YU SM, WANG JX, ZHENG HC, et al. Pathogenesis from inflammation to cancer in NASH-derived HCC[J]. J Hepatocell Carcinoma, 2022, 9: 855- 867. DOI: 10.2147/JHC.S377768. [17] LLOVET JM, WILLOUGHBY CE, SINGAL AG, et al. Nonalcoholic steatohepatitis-related hepatocellular carcinoma: Pathogenesis and treatment[J]. Nat Rev Gastroenterol Hepatol, 2023, 20( 8): 487- 503. DOI: 10.1038/s41575-023-00754-7. [18] van der WINDT DJ, SUD V, ZHANG HJ, et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis[J]. Hepatology, 2018, 68( 4): 1347- 1360. DOI: 10.1002/hep.29914. [19] ZHAO XH, YANG L, CHANG N, et al. Neutrophils undergo switch of apoptosis to NETosis during murine fatty liver injury via S1P receptor 2 signaling[J]. Cell Death Dis, 2020, 11( 5): 379. DOI: 10.1038/s41419-020-2582-1. [20] LIU Y, ZHANG X, CHEN S, et al. Gut-derived lipopolysaccharide promotes alcoholic hepatosteatosis and subsequent hepatocellular carcinoma by stimulating neutrophil extracellular traps through toll-like receptor 4[J]. Clin Mol Hepatol, 2022, 28( 3): 522- 539. DOI: 10.3350/cmh.2022.0039. [21] CAO XT, LAN QS, XU HY, et al. Granulocyte-like myeloid-derived suppressor cells: The culprits of neutrophil extracellular traps formation in the pre-metastatic niche[J]. Int Immunopharmacol, 2024, 143( Pt 3): 113500. DOI: 10.1016/j.intimp.2024.113500. [22] LASSER SA, OZBAY KURT FG, ARKHYPOV I, et al. Myeloid-derived suppressor cells in cancer and cancer therapy[J]. Nat Rev Clin Oncol, 2024, 21( 2): 147- 164. DOI: 10.1038/s41571-023-00846-y. [23] XU M, XU H, LING YW, et al. Neutrophil extracellular traps-triggered hepatocellular senescence exacerbates lipotoxicity in non-alcoholic steatohepatitis[J]. J Adv Res, 2025: S2090-S1232(25)00175- 4. DOI: 10.1016/j.jare.2025.03.015. [24] LI XT, GAO Q, WU WH, et al. FGL2-MCOLN3-autophagy axis-triggered neutrophil extracellular traps exacerbate liver injury in fulminant viral hepatitis[J]. Cell Mol Gastroenterol Hepatol, 2022, 14( 5): 1077- 1101. DOI: 10.1016/j.jcmgh.2022.07.014. [25] HU SN, LIU XW, GAO Y, et al. Hepatitis B virus inhibits neutrophil extracellular trap release by modulating reactive oxygen species production and autophagy[J]. J Immunol, 2019, 202( 3): 805- 815. DOI: 10.4049/jimmunol.1800871. [26] ZHAN X, WU R, KONG XH, et al. Elevated neutrophil extracellular traps by HBV-mediated S100A9-TLR4/RAGE-ROS cascade facilitate the growth and metastasis of hepatocellular carcinoma[J]. Cancer Commun, 2023, 43( 2): 225- 245. DOI: 10.1002/cac2.12388. [27] JIANG ZZ, PENG ZP, LIU XC, et al. Neutrophil extracellular traps induce tumor metastasis through dual effects on cancer and endothelial cells[J]. Oncoimmunology, 2022, 11( 1): 2052418. DOI: 10.1080/2162402X.2022.2052418. [28] YANG C, WEI XQ, ZHENG J, et al. A correlative study between IVIM-DWI parameters and VEGF and MMPs expression in hepatocellular carcinoma[J]. Quant Imaging Med Surg, 2023, 13( 3): 1887- 1898. DOI: 10.21037/qims-22-271. [29] YANG LY, LUO Q, LU L, et al. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response[J]. J Hematol Oncol, 2020, 13( 1): 3. DOI: 10.1186/s13045-019-0836-0. [30] WANG H, ZHANG HJ, WANG Y, et al. Regulatory T-cell and neutrophil extracellular trap interaction contributes to carcinogenesis in non-alcoholic steatohepatitis[J]. J Hepatol, 2021, 75( 6): 1271- 1283. DOI: 10.1016/j.jhep.2021.07.032. [31] YU Y, ZHANG CY, DONG BW, et al. Neutrophil extracellular traps promote immune escape in hepatocellular carcinoma by up-regulating CD73 through Notch2[J]. Cancer Lett, 2024, 598: 217098. DOI: 10.1016/j.canlet.2024.217098. [32] SHEN XT, XIE SZ, ZHENG X, et al. Cirrhotic-extracellular matrix attenuates aPD-1 treatment response by initiating immunosuppressive neutrophil extracellular traps formation in hepatocellular carcinoma[J]. Exp Hematol Oncol, 2024, 13( 1): 20. DOI: 10.1186/s40164-024-00476-9. [33] CHENG YS, GONG YH, CHEN XX, et al. Injectable adhesive hemostatic gel with tumor acidity neutralizer and neutrophil extracellular traps lyase for enhancing adoptive NK cell therapy prevents post-resection recurrence of hepatocellular carcinoma[J]. Biomaterials, 2022, 284: 121506. DOI: 10.1016/j.biomaterials.2022.121506. [34] YANG LY, SHEN XT, SUN HT, et al. Neutrophil extracellular traps in hepatocellular carcinoma are enriched in oxidized mitochondrial DNA which is highly pro-inflammatory and pro-metastatic[J]. J Cancer, 2022, 13( 4): 1261- 1271. DOI: 10.7150/jca.64170. [35] ZHONG WT, WANG QY, SHEN XF, et al. The emerging role of neutrophil extracellular traps in cancer: From lab to ward[J]. Front Oncol, 2023, 13: 1163802. DOI: 10.3389/fonc.2023.1163802. [36] ZHA CJ, MENG XQ, LI LL, et al. Neutrophil extracellular traps mediate the crosstalk between glioma progression and the tumor microenvironment via the HMGB1/RAGE/IL-8 axis[J]. Cancer Biol Med, 2020, 17( 1): 154- 168. DOI: 10.20892/j.issn.2095-3941.2019.0353. [37] ZHU D, LU Y, WANG YM, et al. PAD4 and its inhibitors in cancer progression and prognosis[J]. Pharmaceutics, 2022, 14( 11): 2414. DOI: 10.3390/pharmaceutics14112414. [38] SCHNEIDER AH, MACHADO CC, VERAS FP, et al. Neutrophil extracellular traps mediate joint hyperalgesia induced by immune inflammation[J]. Rheumatology, 2021, 60( 7): 3461- 3473. DOI: 10.1093/rheumatology/keaa794. [39] ZHANG AG, ZOU XM, YANG SF, et al. Effect of NETs/COX-2 pathway on immune microenvironment and metastasis in gastric cancer[J]. Front Immunol, 2023, 14: 1177604. DOI: 10.3389/fimmu.2023.1177604. [40] ZHAO QT, YUE SQ, CUI Z, et al. Potential involvement of the cyclooxygenase-2 pathway in hepatocellular carcinoma-associated angiogenesis[J]. Life Sci, 2007, 80( 5): 484- 492. DOI: 10.1016/j.lfs.2006.09.038. [41] XIA YJ, WANG Y, XIONG Q, et al. Neutrophil extracellular traps promote MASH fibrosis by metabolic reprogramming of HSC[J]. Hepatology, 2025, 81( 3): 947- 961. DOI: 10.1097/HEP.0000000000000762. [42] LI N, ZHENG X, CHEN MR, et al. Deficient DNASE1L3 facilitates neutrophil extracellular traps-induced invasion via cyclic GMP-AMP synthase and the non-canonical NF-κB pathway in diabetic hepatocellular carcinoma[J]. Clin Transl Immunology, 2022, 11( 4): e1386. DOI: 10.1002/cti2.1386. [43] YANG Y, YU SY, LV C, et al. NETosis in tumour microenvironment of liver: From primary to metastatic hepatic carcinoma[J]. Ageing Res Rev, 2024, 97: 102297. DOI: 10.1016/j.arr.2024.102297. [44] MASUCCI MT, MINOPOLI M, del VECCHIO S, et al. The emerging role of neutrophil extracellular traps(NETs) in tumor progression and metastasis[J]. Front Immunol, 2020, 11: 1749. DOI: 10.3389/fimmu.2020.01749. [45] WANG Y, LIU F, CHEN L, et al. Neutrophil extracellular traps(NETs) promote non-small cell lung cancer metastasis by suppressing lncRNA MIR503HG to activate the NF-κB/NLRP3 inflammasome pathway[J]. Front Immunol, 2022, 13: 867516. DOI: 10.3389/fimmu.2022.867516. [46] ALBRENGUES J, SHIELDS MA, NG D, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice[J]. Science, 2018, 361( 6409): eaao4227. DOI: 10.1126/science.aao4227. [47] LIU DH, YANG XY, WANG XY. Neutrophil extracellular traps promote gastric cancer cell metastasis via the NAT10-mediated N4-acetylcytidine modification of SMYD2[J]. Cell Signal, 2024, 116: 111014. DOI: 10.1016/j.cellsig.2023.111014. [48] HUANG H, TOHME S, AL-KHAFAJI AB, et al. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury[J]. Hepatology, 2015, 62( 2): 600- 614. DOI: 10.1002/hep.27841. [49] SEN B, AGGARWAL S, NATH R, et al. Secretome of senescent hepatoma cells modulate immune cell fate by macrophage polarization and neutrophil extracellular traps formation[J]. Med Oncol, 2022, 39( 9): 134. DOI: 10.1007/s12032-022-01732-w. [50] CHEN RX, CUI JF, XU CD, et al. The significance of MMP-9 over MMP-2 in HCC invasiveness and recurrence of hepatocellular carcinoma after curative resection[J]. Ann Surg Oncol, 2012, 19( Suppl 3): S375- S384. DOI: 10.1245/s10434-011-1836-7. [51] KOMOROWICZ E, BALÁZS N, TANKA-SALAMON A, et al. Biorelevant polyanions stabilize fibrin against mechanical and proteolytic decomposition: Effects of polymer size and electric charge[J]. J Mech Behav Biomed Mater, 2020, 102: 103459. DOI: 10.1016/j.jmbbm.2019.103459. [52] LIU YY, PU XY, QIN XY, et al. Neutrophil extracellular traps regulate HMGB1 translocation and kupffer cell M1 polarization during acute liver transplantation rejection[J]. Front Immunol, 2022, 13: 823511. DOI: 10.3389/fimmu.2022.823511. [53] VÁRADY CBS, OLIVEIRA AC, MONTEIRO RQ, et al. Recombinant human DNase I for the treatment of cancer-associated thrombosis: A pre-clinical study[J]. Thromb Res, 2021, 203: 131- 137. DOI: 10.1016/j.thromres.2021.04.028. [54] YIN YZ, DAI HJ, SUN XC, et al. HRG inhibits liver cancer lung metastasis by suppressing neutrophil extracellular trap formation[J]. Clin Transl Med, 2023, 13( 6): e1283. DOI: 10.1002/ctm2.1283. [55] XIN HJ, LAI QW, ZHOU YC, et al. Noninvasive evaluation of neutrophil extracellular traps signature predicts clinical outcomes and immunotherapy response in hepatocellular carcinoma[J]. Front Immunol, 2023, 14: 1134521. DOI: 10.3389/fimmu.2023.1134521. -



 PDF下载 ( 1155 KB)
PDF下载 ( 1155 KB)

 下载:
下载:


