基于共价闭合环状DNA动力学的慢性乙型肝炎治疗策略
DOI: 10.12449/JCH260103
Treatment strategies for chronic hepatitis B based on covalently closed circular DNA dynamics
-
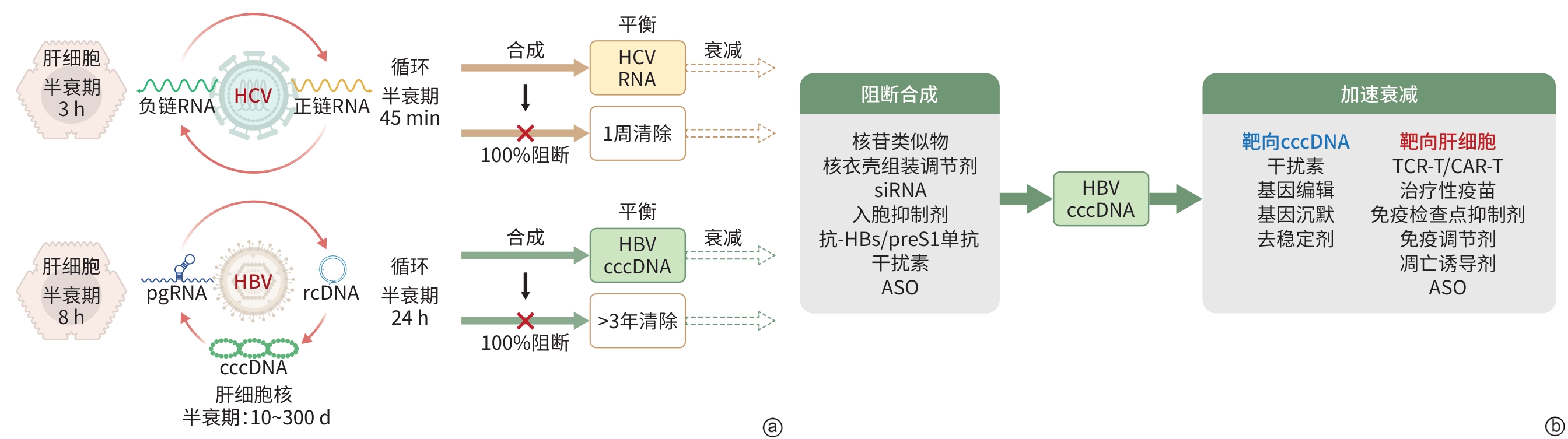
摘要: 实现慢性乙型肝炎病毒(HBV)感染的普遍治愈,是乙型肝炎研究领域的最高目标。深入探索HBV感染治愈的可能途径,对于明确关键研究方向具有重要意义。共价闭合环状DNA(cccDNA)作为HBV复制循环中最难以被清除的遗传物质,既是实现治愈的主要障碍,也是构建治愈策略分析框架的核心基点。本文在概述“cccDNA动力学”思维框架的基础上,进一步阐释其核心内涵,并以此为依据系统探讨论述促进cccDNA衰减的关键策略。Abstract: Cure of chronic hepatitis B virus (HBV) infection is the ultimate goal in HBV-related research, and exploring potential curative pathways may help to identify key research directions. Covalently closed circular DNA (cccDNA), as the most difficult genetic material to be eliminated within the HBV replication cycle, is not only a primary impediment to achieving cure, but also a key issue for establishing an analytical framework for cure strategies. This article reviews the thinking framework of “cccDNA dynamics”, further elaborates on its core implications, and systematically discusses the main strategies to promote cccDNA decay.
-
Key words:
- Hepatitis B Virus /
- Covalently Closed Circular DNA /
- Therapeutics
-
注: a,HBV和HCV复制周期中关键遗传物质的动力学差异导致直接抗病毒药物的疗效差异;b,从cccDNA动力学角度分类抗乙型肝炎药物(部分药物可以发挥两种作用)。HCV,丙型肝炎病毒;HBV,乙型肝炎病毒;rcDNA,松弛双链环状DNA;pgRNA,前基因组RNA;cccDNA,共价闭合环状DNA;siRNA,小干扰RNA;抗-HBs,乙型肝炎病毒表面抗体;preS 1,前S1蛋白;ASO,反义寡核苷酸;TCR-T,T细胞受体工程化T细胞;CAR-T,嵌合抗原受体T细胞。
图 1 基于cccDNA动力学分析慢性乙型肝炎治疗策略
Figure 1. Analysis of treatment strategies for chronic hepatitis B based on cccDNA kinetics
-
[1] LINK JO, TAYLOR JG, XU LH, et al. Discovery of ledipasvir(GS-5885): A potent, once-daily oral NS5A inhibitor for the treatment of hepatitis C virus infection[J]. J Med Chem, 2014, 57( 5): 2033- 2046. DOI: 10.1021/jm401499g. [2] CHATTERJEE A, SMITH PF, PERELSON AS. Hepatitis C viral kinetics: The past, present, and future[J]. Clin Liver Dis, 2013, 17( 1): 13- 26. DOI: 10.1016/j.cld.2012.09.003. [3] NGUYEN THT, GUEDJ J, UPRICHARD SL, et al. The paradox of highly effective sofosbuvir-based combination therapy despite slow viral decline: Can we still rely on viral kinetics?[J]. Sci Rep, 2017, 7( 1): 10233. DOI: 10.1038/s41598-017-09776-z. [4] NOWAK MA, BONHOEFFER S, HILL AM, et al. Viral dynamics in hepatitis B virus infection[J]. Proc Natl Acad Sci USA, 1996, 93( 9): 4398- 4402. DOI: 10.1073/pnas.93.9.4398. [5] IMAM H, KHAN M, GOKHALE NS, et al. N6-methyladenosine modification of hepatitis B virus RNA differentially regulates the viral life cycle[J]. Proc Natl Acad Sci USA, 2018, 115( 35): 8829- 8834. DOI: 10.1073/pnas.1808319115. [6] LIU F, LEE ACH, GUO F, et al. Host poly(A) polymerases PAPD5 and PAPD7 provide two layers of protection that ensure the integrity and stability of hepatitis B virus RNA[J]. J Virol, 2021, 95( 18): e00574-21. DOI: 10.1128/JVI.00574-21. [7] TABATA K, NEUFELDT CJ, BARTENSCHLAGER R. Hepatitis C virus replication[J]. Cold Spring Harb Perspect Med, 2020, 10( 3): a037093. DOI: 10.1101/cshperspect.a037093. [8] MATA M, NEBEN S, MAJZOUB K, et al. Impact of a patient-derived hepatitis C viral RNA genome with a mutated microRNA binding site[J]. PLoS Pathog, 2019, 15( 5): e1007467. DOI: 10.1371/journal.ppat.1007467. [9] HU JL, HUANG AL. A conceptual framework for dynamics of cccDNA in hepatitis B virus[J]. Chin J Hepatol, 2023, 31( 5): 545- 550. DOI: 10.3760/cma.j.cn501113-20230330-00134.胡接力, 黄爱龙. 乙型肝炎病毒cccDNA动力学的思维框架[J]. 中华肝脏病杂志, 2023, 31( 5): 545- 550. DOI: 10.3760/cma.j.cn501113-20230330-00134. [11] HU JL, HUANG AL. Classifying hepatitis B therapies with insights from covalently closed circular DNA dynamics[J]. Virol Sin, 2024, 39( 1): 9- 23. DOI: 10.1016/j.virs.2023.12.005. [12] ASABE S, WIELAND SF, CHATTOPADHYAY PK, et al. The size of the viral inoculum contributes to the outcome of hepatitis B virus infection[J]. J Virol, 2009, 83( 19): 9652- 9662. DOI: 10.1128/JVI.00867-09. [13] GUIDOTTI LG, ROCHFORD R, CHUNG J, et al. Viral clearance without destruction of infected cells during acute HBV infection[J]. Science, 1999, 284( 5415): 825- 829. DOI: 10.1126/science.284.5415.825. [14] HU JL, HUANG AL. Chronic infection of hepatitis B virus and cellular immunity[J]. Chin J Microbiol Immunol, 2023, 43( 7): 494- 501. DOI: 10.3760/cma.j.cn112309-20230619-00179.胡接力, 黄爱龙. 慢性乙型肝炎病毒感染与细胞免疫[J]. 中华微生物学和免疫学杂志, 2023, 43( 7): 494- 501. DOI: 10.3760/cma.j.cn112309-20230619-00179. [15] FRELIN L, WAHLSTRÖM T, TUCKER AE, et al. A mechanism to explain the selection of the hepatitis E antigen-negative mutant during chronic hepatitis B virus infection[J]. J Virol, 2009, 83( 3): 1379- 1392. DOI: 10.1128/JVI.01902-08. [16] VOLZ T, LUTGEHETMANN M, WACHTLER P, et al. Impaired intrahepatic hepatitis B virus productivity contributes to low viremia in most HBeAg-negative patients[J]. Gastroenterology, 2007, 133( 3): 843- 852. DOI: 10.1053/j.gastro.2007.06.057. [17] WALKER A, SCHWARZ T, BRINKMANN-PAULUKAT J, et al. Immune escape pathways from the HBV core(18-27) CD8 T cell response are driven by individual HLA class I alleles[J]. Front Immunol, 2022, 13: 1045498. DOI: 10.3389/fimmu.2022.1045498. [18] LI GX, YANG DL, LIU X, et al. Precore mutation enhances viral replication to facilitate persistent infection especially in HBeAg-negative patients[J]. Virol Sin, 2024, 39( 2): 319- 330. DOI: 10.1016/j.virs.2024.03.003. [19] UCHIDA T, IMAMURA M, HAYES CN, et al. HBV with precore and basal core promoter mutations exhibits a high replication phenotype and causes ER stress-mediated cell death in humanized liver chimeric mice[J]. Hepatology, 2023, 78( 3): 929- 942. DOI: 10.1097/HEP.0000000000000335. [20] ROSSI M, VECCHI A, TIEZZI C, et al. Phenotypic CD8 T cell profiling in chronic hepatitis B to predict HBV-specific CD8 T cell susceptibility to functional restoration in vitro[J]. Gut, 2023, 72( 11): 2123- 2137. DOI: 10.1136/gutjnl-2022-327202. [21] HOOGEVEEN RC, ROBIDOUX MP, SCHWARZ T, et al. Phenotype and function of HBV-specific T cells is determined by the targeted epitope in addition to the stage of infection[J]. Gut, 2019, 68( 5): 893- 904. DOI: 10.1136/gutjnl-2018-316644. [22] BAULU E, GARDET C, CHUVIN N, et al. TCR-engineered T cell therapy in solid tumors: State of the art and perspectives[J]. Sci Adv, 2023, 9( 7): eadf3700. DOI: 10.1126/sciadv.adf3700. [23] HU JL, JI YW, PENG P, et al. Clinical cure and safe drug withdrawal in chronic hepatitis B[J]. Chin J Hepatol, 2025, 33( 6): 526- 533. DOI: 10.3760/cma.j.cn501113-20250106-00008.胡接力, 季业伟, 彭湃, 等. 慢性乙型肝炎临床治愈与安全停药[J]. 中华肝脏病杂志, 2025, 33( 6): 526- 533. DOI: 10.3760/cma.j.cn501113-20250106-00008. [24] WU XA, QUAN DM, LI W, et al. Clinical results of an HBV-specific T-cell receptor-T-cell therapy(SCG101) in patients with HBV-related hepatocellular carcinoma treated in an investigator-initiated, interventional trial[J]. Gut, 2025, 75( 1): 147- 160. DOI: 10.1136/gutjnl-2025-335456. [25] WAN XS, WISSKIRCHEN K, JIN T, et al. Genetically-modified, redirected T cells target hepatitis B surface antigen-positive hepatocytes and hepatocellular carcinoma lesions in a clinical setting[J]. Clin Mol Hepatol, 2024, 30( 4): 735- 755. DOI: 10.3350/cmh.2024.0058. [26] JIN X, LI L. New advances in tree shrew model in experimental studies of hepatitis B virus[J]. J Clin Hepatol, 2015, 31( 9): 1524- 1527. DOI: 10.3969/j.issn.1001-5256.2015.09.041.金雄, 李立. 乙型肝炎树鼩模型的研究进展[J]. 临床肝胆病杂志, 2015, 31( 9): 1524- 1527. DOI: 10.3969/j.issn.1001-5256.2015.09.041. [27] WALTER E, KEIST R, NIEDERÖST B, et al. Hepatitis B virus infection of Tupaia hepatocytes in vitro and in vivo[J]. Hepatology, 1996, 24( 1): 1- 5. DOI: 10.1002/hep.510240101. [28] YUEN MF, HEO J, KUMADA H, et al. Phase IIa, randomised, double-blind study of GSK3389404 in patients with chronic hepatitis B on stable nucleos(t)ide therapy[J]. J Hepatol, 2022, 77( 4): 967- 977. DOI: 10.1016/j.jhep.2022.05.031. [29] JOSHI S, FREUDENBERG JM, SINGH JM, et al. Immunomodulation by bepirovirsen may induce killing of infected hepatocytes(B-Together study)[J]. Hepatol Int, 2025. DOI: 10.1007/s12072-025-10917-0.[ Epub ahead of print] [30] YUEN MF, LIM SG, PLESNIAK R, et al. Efficacy and safety of bepirovirsen in chronic hepatitis B infection[J]. N Engl J Med, 2022, 387( 21): 1957- 1968. DOI: 10.1056/NEJMoa2210027. -



 PDF下载 ( 957 KB)
PDF下载 ( 957 KB)

 下载:
下载:


