乙型肝炎病毒基因组整合的研究进展
DOI: 10.12449/JCH260104
-
摘要: 乙型肝炎病毒(HBV) DNA整合是慢性乙型肝炎(CHB)实现功能性治愈和阻断肝癌发生所必须克服的核心障碍。在病毒逆转录过程中,占总量5%~10%双链线性DNA随机插入宿主染色体,形成持续存在的整合DNA(iDNA)并持续表达乙型肝炎病毒表面抗原,驱动B细胞与T细胞免疫耗竭,从而维持机体的免疫耐受状态。整合DNA贯穿整个感染自然史,并可能通过增强子促进潜能不确定的克隆性增生,逐步累积癌前突变,最终发展为肝细胞癌。长期核苷(酸)类似物或干扰素治疗虽可抑制病毒复制,减少HBV DNA整合,但现有手段仍难以彻底清除既有的iDNA。因此,未来亟需发展能够精准靶向整合断点、表观遗传沉默iDNA或清除整合克隆的创新策略,从而显著提升CHB的功能性治愈率,并从根源上降低肝细胞癌的发生风险。Abstract: HBV DNA integration (iDNA) is the core barrier that must be overcome to achieve functional cure for chronic hepatitis B (CHB) and to prevent the occurrence of hepatocellular carcinoma (HCC). During reverse transcription, 5% — 10% of viral genomes are converted into double-stranded linear DNA that is randomly inserted into host chromosomes, generating stable iDNA and continuously producing HBsAg, thereby driving B- and T-cell immune exhaustion and locking the host in an immune-tolerant state. The process of iDNA runs throughout the entire natural history of HBV infection, and the viral enhancers it carries can promote clonal hyperplasia of indeterminate potential, accumulate pre-neoplastic mutations, and ultimately lead to HCC. Although long-term nucleos(t)ide analog or interferon therapy can suppress viral replication and reduce the formation of new integrations, existing therapies still fail to eliminate existing iDNA. Therefore, there is an urgent need for innovative strategies that can precisely target integration breakpoints, epigenetically silence iDNA, or eradicate integrated clones, so as to significantly increase the functional cure rate of CHB and fundamentally reduce the risk of HCC.
-
Key words:
- Chronic Hepatitis B /
- HBV DNA Integration /
- Carcinoma, Hepatocellular
-
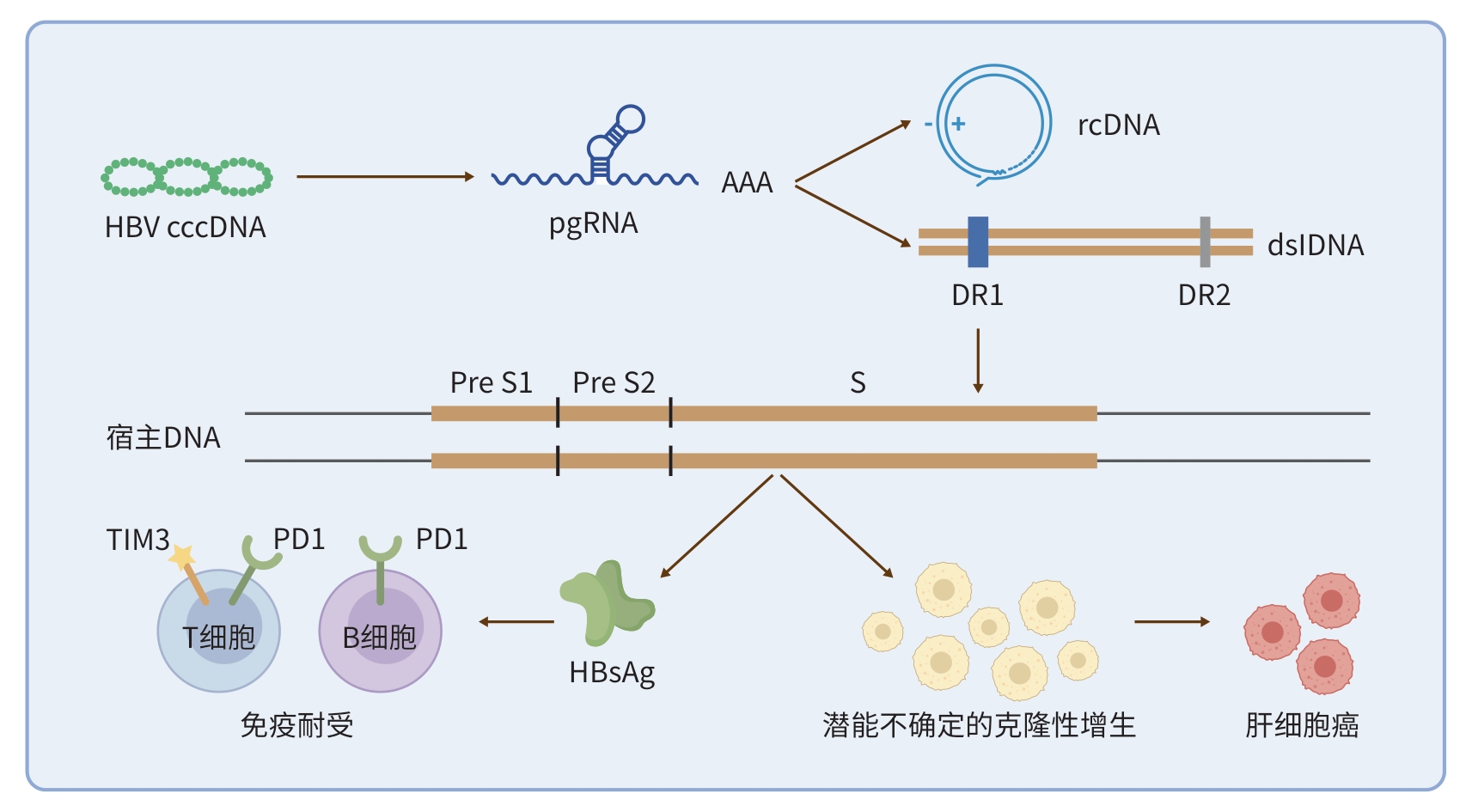
注: HBV cccDNA首先转录为pgRNA;随后,pgRNA经逆转录主要形成rcDNA,同时少量产生dslDNA。dslDNA可随机插入宿主基因组,产生双重后果:一方面,整合片段持续翻译大量HBsAg,诱导T细胞与B细胞进入免疫耐受状态;另一方面,携带iDNA的肝细胞获得潜能未定的克隆性增殖优势,逐步进展为HCC。cccDNA,共价闭合环状DNA;pgRNA,前基因组RNA;rcDNA,松弛环状双链DNA;DR,直接重复序列;dslDNA,双链线性DNA; HbsAg,乙型肝炎表面抗原;PreS1/S2,前S1/S2;TIM3,T细胞免疫球蛋白黏蛋白-3;PD1,程序性死亡受体1;AAA,poly(A)尾。
图 1 HBV DNA整合的机制及作用
Figure 1. Mechanisms and effects of HBV DNA integration
-
[1] YAN RY, SUN MH, YANG HY, et al. 2024 latest report on hepatitis B virus epidemiology in China: Current status, changing trajectory, and challenges[J]. Hepatobiliary Surg Nutr, 2025, 14( 1): 66- 77. DOI: 10.21037/hbsn-2024-754. [2] MA LL, CHEN SZ, WANG HY, et al. Hepatitis B virus integration and hepatocarcinogenesis[J]. Liver Res, 2025, 9( 3): 189- 198. DOI: 10.1016/j.livres.2025.09.002. [3] ZHANG MM, CHEN H, LIU H, et al. The impact of integrated hepatitis B virus DNA on oncogenesis and antiviral therapy[J]. Biomark Res, 2024, 12( 1): 84. DOI: 10.1186/s40364-024-00611-y. [4] BOUSALI M, PAPATHEODORIDIS G, PARASKEVIS D, et al. Hepatitis B virus DNA integration, chronic infections and hepatocellular carcinoma[J]. Microorganisms, 2021, 9( 8): 1787. DOI: 10.3390/microorganisms9081787. [5] ZOULIM F, CHEN PJ, DANDRI M, et al. Hepatitis B virus DNA integration: Implications for diagnostics, therapy, and outcome[J]. J Hepatol, 2024, 81( 6): 1087- 1099. DOI: 10.1016/j.jhep.2024.06.037. [6] GU ZQ, JIANG QQ, ABULAITI A, et al. Hepatitis B virus enhancer 1 activates preS1 and preS2 promoters of integrated HBV DNA impairing HBsAg secretion[J]. JHEP Rep, 2024, 6( 9): 101144. DOI: 10.1016/j.jhepr.2024.101144. [7] POLLICINO T, CAMINITI G. HBV-integration studies in the clinic: Role in the natural history of infection[J]. Viruses, 2021, 13( 3): 368. DOI: 10.3390/v13030368. [8] CHEN Y, DONG Y, WEI SZ, et al. Genomic integration of hepatitis B virus into human hepatocytes in early childhood cirrhosis[J]. Liver Int, 2025, 45( 4): e70080. DOI: 10.1111/liv.70080. [9] KIMBI GC, KRAMVIS A, KEW MC. Integration of hepatitis B virus DNA into chromosomal DNA during acute hepatitis B[J]. World J Gastroenterol, 2005, 11( 41): 6416- 6421. DOI: 10.3748/wjg.v11.i41.6416. [10] LAU DT, KIM ES, WANG ZL, et al. Differential intrahepatic integrated HBV DNA patterns between HBeAg-positive and HBeAg-negative chronic hepatitis B[J]. medRxiv, 2025. DOI: 10.1101/2025.02.28.25322668.[Preprint] [11] LI CL, HSU CL, LIN YY, et al. HBV DNA integration into telomerase or MLL4 genes and TERT promoter point mutation as three independent signatures in subgrouping HBV-related HCC with distinct features[J]. Liver Cancer, 2024, 13( 1): 41- 55. DOI: 10.1159/000530699. [12] LI MG, WU SS, LUO HQ, et al. HBV DNA integration gene CCDC91 is oncogenic and a potential therapeutic target for hepatocellular carcinoma[J]. Commun Biol, 2025, 8( 1): 1079. DOI: 10.1038/s42003-025-08369-1. [13] BURTON AR, PALLETT LJ, MCCOY LE, et al. Circulating and intrahepatic antiviral B cells are defective in hepatitis B[J]. J Clin Invest, 2018, 128( 10): 4588- 4603. DOI: 10.1172/JCI121960. [14] YU F, ZHU Y, LI SH, et al. Dysfunction and regulatory interplay of T and B cells in chronic hepatitis B: Immunotherapy and emerging antiviral strategies[J]. Front Cell Infect Microbiol, 2024, 14: 1488527. DOI: 10.3389/fcimb.2024.1488527. [15] MARRAPU S, SONI JR, KAMAL K, et al. Hepatitis B functional cure: Current and future perspective[J]. World J Hepatol, 2025, 17( 10): 110107. DOI: 10.4254/wjh.v17.i10.110107. [16] PAN DZ, SOULETTE CM, AGGARWAL A, et al. Effects of tenofovir disoproxil fumarate on intrahepatic viral burden and liver immune microenvironment in patients with chronic hepatitis B[J]. Gut, 2025, 74( 4): 628- 638. DOI: 10.1136/gutjnl-2024-332526. [17] ZHANG MY, ZHANG HK, CHENG XM, et al. Liver biopsy of chronic hepatitis B patients indicates HBV integration profile may complicate the endpoint and effect of entecavir treatment[J]. Antiviral Res, 2022, 204: 105363. DOI: 10.1016/j.antiviral.2022.105363. [18] TADDESE M, GRUDDA T, BELLUCCINI G, et al. Transcription of hepatitis B surface antigen shifts from cccDNA to integrated HBV DNA during treatment[J]. J Clin Invest, 2025, 135( 6): e184243. DOI: 10.1172/JCI184243. [19] GAO N, GUAN GW, XU GL, et al. Integrated HBV DNA and cccDNA maintain transcriptional activity in intrahepatic HBsAg-positive patients with functional cure following PEG-IFN-based therapy[J]. Aliment Pharmacol Ther, 2023, 58( 10): 1086- 1098. DOI: 10.1111/apt.17670. [20] KILANY MM, SONNEVELD MJ, FELD JJ, et al. Management of immune-tolerant chronic hepatitis B[J]. Hepatology, 2025. DOI: 10.1097/HEP.0000000000001407. DOI: 10.1097/HEP.0000000000001407. [21] YUEN MF, WONG DK, SCHLUEP T, et al. Long-term serological, virological and histological responses to RNA inhibition by ARC-520 in Chinese chronic hepatitis B patients on entecavir treatment[J]. Gut, 2022, 71( 4): 789- 797. DOI: 10.1136/gutjnl-2020-323445. [22] YUEN MF, LIM SG, PLESNIAK R, et al. Efficacy and safety of bepirovirsen in chronic hepatitis B infection[J]. N Engl J Med, 2022, 387( 21): 1957- 1968. DOI: 10.1056/nejmoa2210027. -



 PDF下载 ( 627 KB)
PDF下载 ( 627 KB)

 下载:
下载:


