失代偿期肝硬化患者再代偿的影响因素及预后分析
DOI: 10.12449/JCH260111
Influencing factors for recompensation and its impact on the prognosis in patients with decompensated liver cirrhosis
-
摘要:
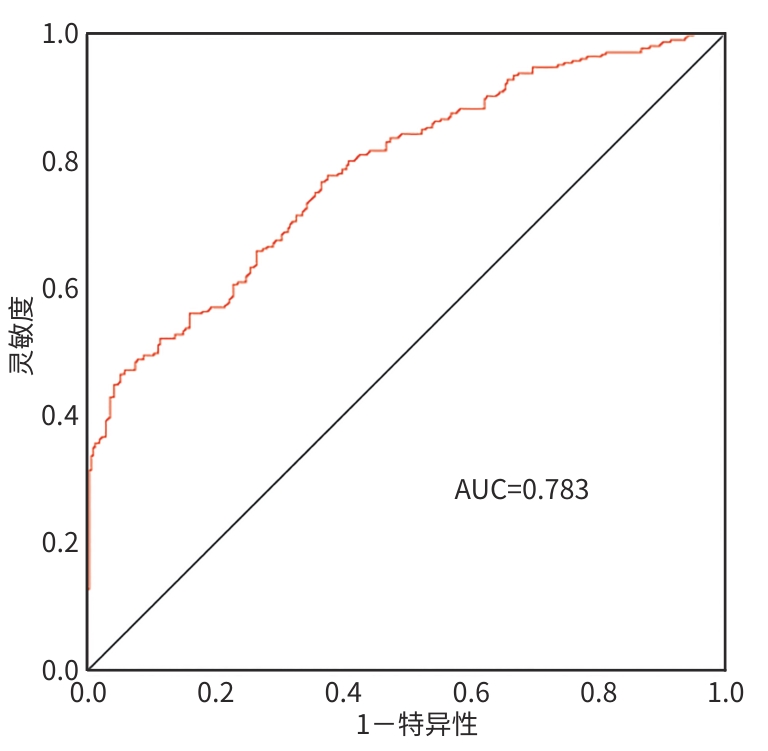
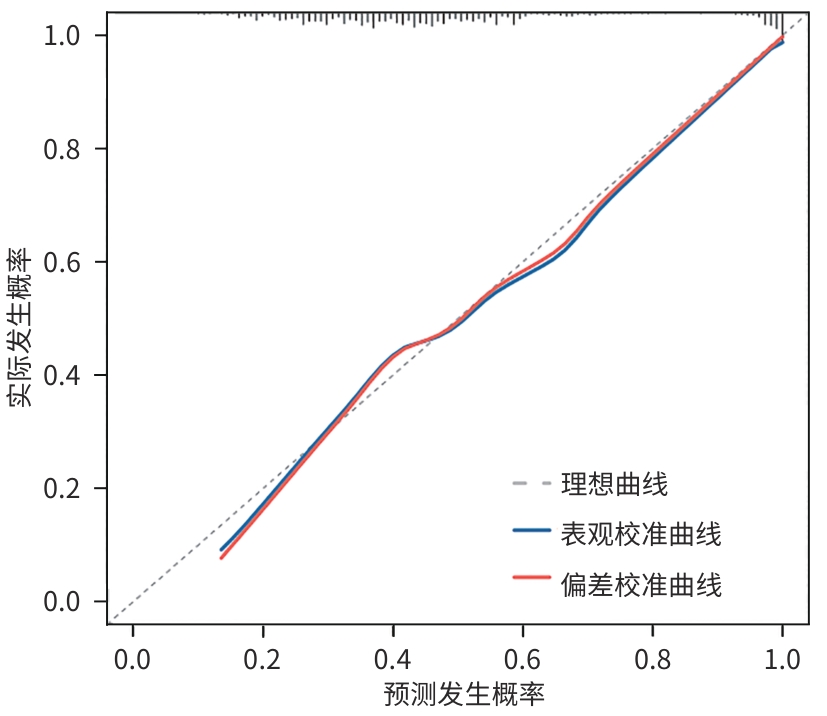
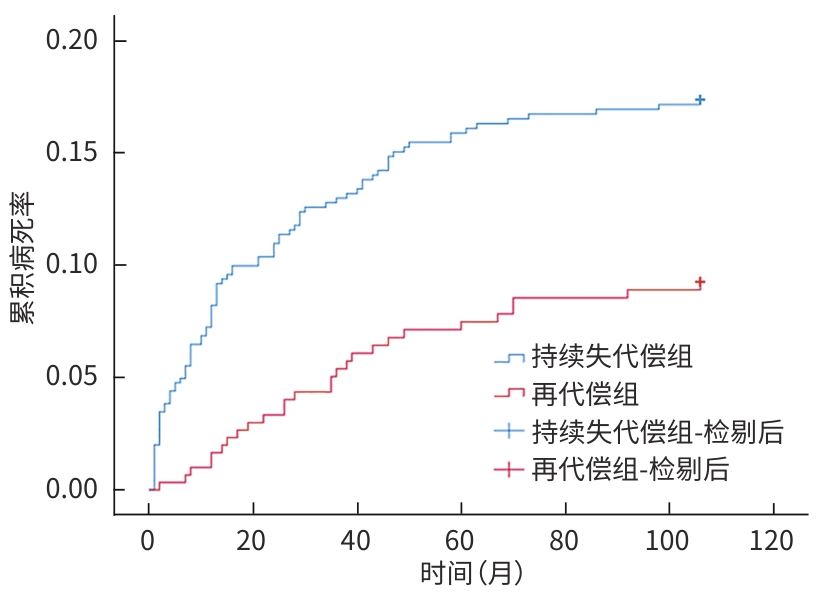
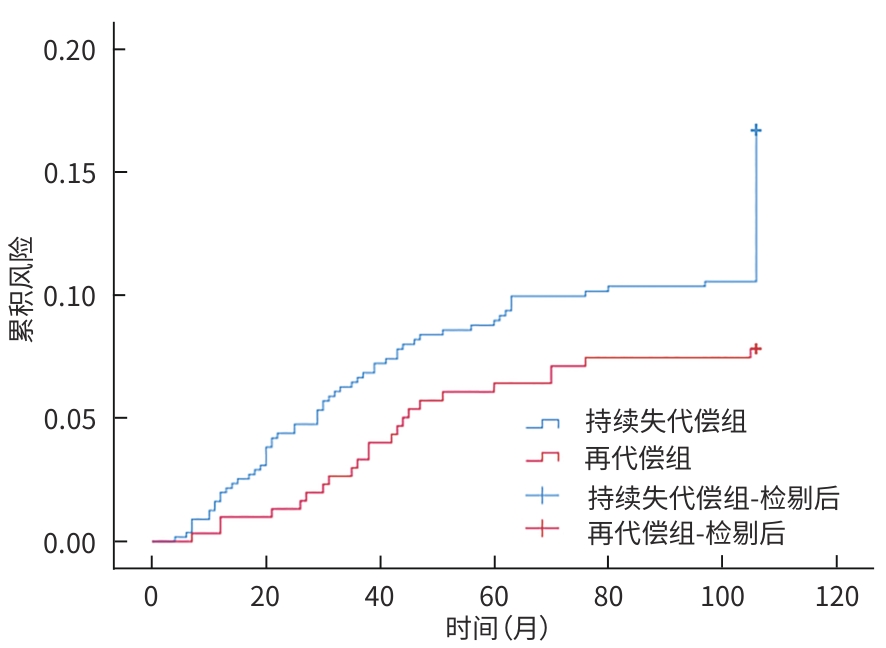
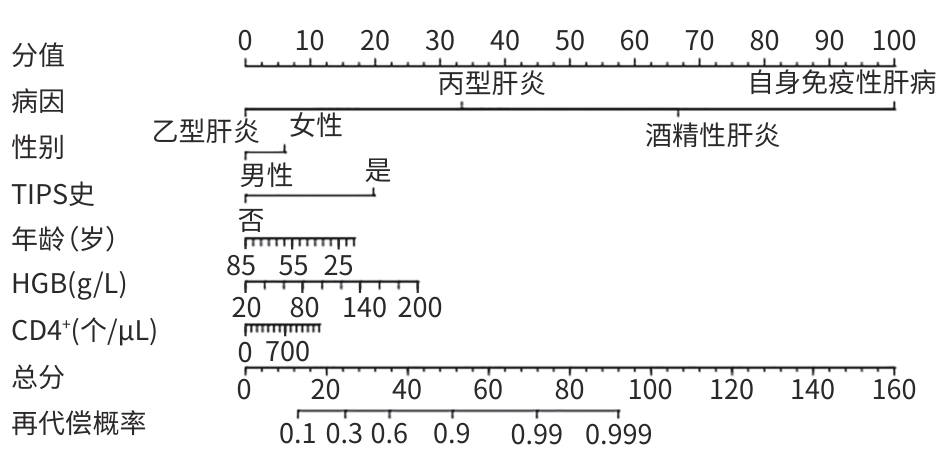
目的 探讨失代偿期肝硬化患者再代偿发生的影响因素,评估再代偿发生对预后的影响,为临床早期识别中高危患者提供依据。 方法 回顾性收集2016年1月—2022年12月就诊于昆明市第三人民医院诊断为乙型肝炎、丙型肝炎、酒精性肝炎及自身免疫性肝炎相关失代偿期肝硬化患者的临床资料,将患者分为再代偿组和持续失代偿组。为控制混杂因素,以是否发生再代偿作为分组变量,以体重指数、饮酒史、人类免疫缺陷病毒感染史、甘油三酯、总胆固醇、低密度脂蛋白、高密度脂蛋白为协变量,计算倾向性评分,并采用卡钳值0.1进行1∶1最邻近匹配,经倾向性评分匹配(PSM)后,得到协变量相对均衡的再代偿组与持续失代偿组。采用单因素及多因素Cox比例风险回归模型分析再代偿影响因素,使用“rms”程序包构建列线图,绘制受试者操作特征曲线并计算曲线下面积,使用Hosmer-Lemeshow检验评估模型的拟合度,使用“Calibration Curves”程序包绘制校准曲线对模型进行评价。Kaplan-Meier法绘制生存曲线,采用Log-rank法进行组间比较。 结果 863例失代偿期肝硬化患者有305例发生再代偿,发生率为35.3%。PSM后,610例配对成功,再代偿组与持续失代偿组各305例。单因素及多因素Cox回归分析结果显示,病因[丙型肝炎(HR=0.288,P=0.002)]、男性(HR=0.701,P=0.016)、年龄(HR=0.988,P=0.047)、血红蛋白(HGB)(HR=1.006,P=0.017)、CD4 T淋巴细胞(HR=1.001,P=0.047)、经颈静脉肝内门体分流术(TIPS)(HR=1.808,P=0.024)是失代偿期肝硬化患者再代偿的独立影响因素。随访期间,116例患者发生肝病相关死亡,其中,再代偿组27例(8.85%),持续失代偿组89例(15.95%);109例患者发生肝细胞癌(HCC),其中,再代偿组23例(7.54%),持续失代偿组86例(15.41%)。Kaplan-Meier生存曲线显示,不同代偿状态患者肝病相关病死率及HCC发生率曲线明显分离,Log-rank检验提示两组患者肝病相关病死率(χ2=9.023,P=0.003)及HCC发生率(χ2=10.526,P=0.001)差异均有统计学意义。 结论 病因、性别、年龄、TIPS史、HGB、CD4 T淋巴细胞是失代偿期肝硬化患者再代偿的独立影响因素,不同病因的失代偿期肝硬化患者再代偿的发生率存在显著差异,有TIPS史、女性、低年龄、高HGB水平及高CD4 T淋巴细胞水平的失代偿期肝硬化患者更容易出现再代偿。实现再代偿是改善患者长期预后的关键,可明显降低患者长期肝病相关病死率及HCC发生率。 Abstract:Objective To investigate the influencing factors for recompensation in patients with decompensated liver cirrhosis, as well as the impact of recompensation on the prognosis of such patients, and to provide a basis for early identification of high-risk patients in clinical practice. Methods A retrospective analysis was performed for the clinical data of patients who attended The Third People’s Hospital of Kunming from January 2016 to December 2022 and were diagnosed with decompensated liver cirrhosis due to hepatitis B, hepatitis C, alcoholic hepatitis, and autoimmune hepatitis, and they were divided into recompensation group and persistent decompensation group. To control for confounding factors, whether recompensation occurred was used as the rouping variable,and BMI, alcohol consumption history, HIV infection history, TG, CHOL, LDL, and HDL were used as covariates. The propensity score was calculated, and 1:1 nearest neighbor matching was performed with a caliper value of 0.1. After propensity score matching, the recompensation group and the persistent decompensation group with relatively balanced covariates were obtained. Univariate and multivariate Cox proportional-hazards regression model analyses were used to investigate the influencing factors for recompensation; the “rms” package was used to establish a nomogram; the receiver operating characteristic (ROC) curve was plotted to calculate the area under the ROC curve (AUC); the Hosmer-Lemeshow test was used to assess the goodness of fit of the model; the “Calibration Curves” package was used to plot calibration curves for model assessment. The Kaplan-Meier method was used to plot survival curves, and the Log-rank test was used for comparison of survival curves. Results Among the 863 patients with decompensated liver cirrhosis, 305 experienced recompensation, resulting in an incidence rate of 35.3%. After PSM, 610 cases were successfully matched, with 305 cases in each group. The univariate and multivariate Cox regression analyses showed that etiology (hepatitis C: hazard ratio[HR]=0.288, P=0.002); male(HR=0.701, P=0.016), age(HR=0.988, P=0.047), hemoglobin (HGB)(HR=1.006, P=0.017), and CD4 T cell(HR=1.001,P=0.047), TIPS procedure (HR=1.808,P=0.042) were independent influencing factors for recompensation in patients with decompensated liver cirrhosis. During follow-up, 116 patients died of liver disease-related causes, with 27 patients (8.85%) in the recompensation group and 89 (15.95%) in the persistent decompensation group; 109 patients developed HCC, with 23 patients (7.54%) in the recompensation group and 86 (15.41%) in the persistent decompensation group. The Kaplan-Meier survival curves showed significant separation between the patients with different states of compensation in terms of liver disease-related mortality rate and the incidence rate of HCC, and the Log-rank test showed that there were significant differences between the two groups in liver disease-related mortality rate (χ2=9.023, P=0.003) and the incidence rate of HCC (χ2=10.526, P=0.001). Conclusion Etiology,sex,age,TIPS,HGB,and CD4 T cell are independent influencing factors for recompensation in patients with decompensated liver cirrhosis. There is a significant difference in the incidence rate of recompensation between decompensated liver cirrhosis patients with different etiologies, and female patients and patients with a younger age,a history of TIPS, a higher HGB level, and a higher CD4 lymphocyte count are more likely to experience recompensation. Recompensation is the key to improving the long-term prognosis of patients and can significantly reduce long-term liver disease-related mortality rate and the incidence rate of HCC. -
Key words:
- Liver Cirrhosis /
- Risk Factor /
- Prognosis
-
表 1 PSM前再代偿组与持续失代偿组患者的基线资料比较
Table 1. Comparison of baseline data between patients with recovered compensation and those with persistent decompensated before DSM
指标 再代偿组(n=305) 持续失代偿组(n=558) 统计值 P值 男性[例(%)] 205(67.2) 407(72.9) χ2=3.135 0.077 2型糖尿病史[例(%)] 47(15.4) 83(14.9) χ2=0.044 0.834 高血压病史[例(%)] 44(14.4) 69(12.4) χ2=0.736 0.391 饮酒史[例(%)] 116(38.0) 271(48.6) χ2=8.847 0.003 吸烟史[例(%)] 117(38.4) 244(43.7) χ2=2.334 0.127 HIV感染史[例(%)] 8(2.6) 32(5.7) χ2=4.320 0.038 TIPS史[例(%)] 17(5.6) 7(1.3) χ2=13.608 <0.001 PSE史[例(%)] 33(10.8) 32(5.7) χ2=7.321 0.007 内镜治疗史[例(%)] 48(15.7) 90(16.1) χ2=0.022 0.881 口服NSBB[例(%)] 38(12.5) 36(6.5) χ2=9.078 0.003 HE[例(%)] 10(3.3) 41(7.3) χ2=5.872 0.015 感染[例(%)] 94(30.8) 221(39.6) χ2=6.568 0.010 病因治疗[例(%)] 288(93.8) 446(79.9) χ2=29.348 <0.001 失代偿事件[例(%)] χ2=2.999 0.083 1种 279(91.5) 488(87.6) ≥2种 26(8.5) 69(12.4) 病因[例(%)] χ2=12.592 0.006 乙型肝炎 207(67.9) 310(55.6) 丙型肝炎 63(20.7) 154(27.6) 酒精性肝炎 25(8.2) 66(11.8) 自身免疫性肝病 10(3.3) 28(5.0) 腹水分级[例(%)] χ2=25.743 <0.001 无 51(16.7) 59(10.6) 少量 153(50.2) 236(42.3) 中量 76(24.9) 153(27.4) 大量 25(8.2) 110(19.7) Child-Pugh分级[例(%)] χ2=38.966 <0.001 A级 75(24.6) 54(9.7) B级 147(48.2) 279(50.0) C级 83(27.2) 225(40.3) MELD评分[例(%)] χ2=2.128 0.345 低危 108(35.4) 203(36.4) 中危 64(21.0) 137(24.6) 高危 133(43.6) 218(39.1) 年龄(岁) 51.00(44.00~57.00) 51.00(44.75~58.25) Z=-1.346 0.178 BMI(kg/m2) 22.56(21.44~24.28) 22.77(21.69~24.51) Z=-1.362 0.173 WBC(×109/L) 3.93(3.02~5.56) 4.06(2.86~5.68) Z=-0.101 0.919 HGB(g/L) 127.00(107.50~142.00) 115.00(91.00~133.25) Z=-6.005 <0.001 PLT(×109/L) 83.00(55.00~116.20) 73.00(52.00~106.25) Z=-2.502 0.012 TBil(mmol/L) 31.80(21.45~53.55) 35.25(21.48~66.63) Z=-0.940 0.347 AST(U/L) 58.00(37.00~109.50) 56.50(38.75~109.50) Z=-0.123 0.902 ALT(U/L) 39.00(25.00~52.80) 37.00(25.00~65.00) Z=-1.754 0.080 TP(g/L) 63.63±8.94 61.72±9.16 t=2.956 0.597 Alb(g/L) 32.00(28.05~37.15) 28.50(24.70~32.90) Z=-7.964 <0.001 TG(mmol/L) 0.82(0.60~1.15) 0.80(0.55~1.13) Z=-1.075 0.282 CHOL(mmol/L) 3.38(2.72~3.97) 3.12(2.39~3.79) Z=-3.807 <0.001 HDL(mmol/L) 0.95(0.61~1.25) 0.81(0.46~1.15) Z=-3.799 <0.001 LDL(mmol/L) 1.90(1.45~2.30) 1.73(1.24~2.25) Z=-3.146 <0.001 hs-CRP(mg/L) 3.46(1.22~10.11) 5.79(1.74~14.67) Z=-3.774 <0.001 IL-6(pg/mL) 12.61(8.72~23.60) 18.50(10.38~34.37) Z=-4.791 <0.001 PT (s) 16.70(15.30~18.50) 17.20(15.60~19.40) Z=-3.145 0.002 CD4+(个/μL) 461.90(313.30~620.73) 405.05(278.37~562.80) Z=-3.419 0.001 注:HIV,人类免疫缺陷病毒;TIPS,经颈静脉肝内门体静脉分流术;PSE,部分脾动脉栓塞术;NSBB,β受体阻滞剂;HE,肝性脑病;Child-Pugh分级,蔡尔德-皮尤分级;MELD,终末期肝病模型;BMI,体重指数;WBC,白细胞;HGB,血红蛋白;PLT,血小板;TBil,总胆红素;AST,天冬氨酸氨基转移酶;ALT,丙氨酸氨基转移酶;TP,总蛋白;Alb,白蛋白;TG,甘油三酯;CHOL,总胆固醇;HDL,高密度脂蛋白;LDL,低密度脂蛋白;hs-CRP,超敏C反应蛋白;IL-6,白细胞介素6;PT,凝血酶原时间;CD4+,CD4 T淋巴细胞计数。
表 2 PSM 后再代偿组与持续失代偿组患者的基线资料比较
Table 2. Comparison of baseline data between pateints with recovered compensation and those with persistent decompensation after PSM
指标 再代偿组(n=305) 持续失代偿组(n=305) 统计值 P值 男性[例(%)] 205(67.2) 232(76.1) χ2=5.882 0.015 2型糖尿病史[例(%)] 47(15.4) 42(13.8) χ2=0.329 0.566 高血压病史[例(%)] 44(14.4) 25(8.2) χ2=5.899 0.015 饮酒史[例(%)] 116(38.0) 110(36.1) χ2=0.253 0.615 吸烟史[例(%)] 117(38.4) 100(32.8) χ2=2.067 0.151 HIV感染史[例(%)] 8(2.6) 3(1.0) χ2=2.314 0.128 TIPS史[例(%)] 17(5.6) 2(0.7) 0.001 PSE史[例(%)] 33(10.8) 19(6.2) χ2=4.120 0.059 内镜治疗史[例(%)] 48(15.7) 56(18.4) χ2=0.742 0.389 口服NSBB[例(%)] 38(12.5) 22(7.2) χ2=4.732 0.030 HE[例(%)] 10(3.3) 22(7.2) χ2=4.749 0.029 感染[例(%)] 94(30.8) 135(44.3) χ2=11.753 0.001 病因治疗[例(%)] 286(93.8) 277(90.8) χ2=1.867 0.172 失代偿事件[例(%)] χ2=1.475 0.224 1种 279(91.5) 270(88.5) ≥2种 26(8.5) 35(11.5) 病因[例(%)] χ2=130.442 <0.001 乙型肝炎 207(67.9) 303(99.3) 丙型肝炎 63(20.7) 2(0.7) 酒精性肝炎 25(8.2) 0(0.0) 自身免疫性肝病 10(3.3) 0(0.0) 腹水分级[例(%)] χ2=32.418 <0.001 无 51(16.7) 14(4.6) 少量 153(50.2) 158(51.8) 中量 76(24.9) 78(25.6) 大量 25(8.2) 55(18.0) Child-Pugh分级[例(%)] χ2=24.283 <0.001 A级 75(24.6) 34(11.1) B级 147(48.2) 145(47.5) C级 83(27.2) 126(41.3) MELD评分[例(%)] χ2=47.261 <0.001 低危 108(35.4) 36(11.8) 中危 64(21.0) 83(27.2) 高危 133(43.6) 186(61.0) 年龄(岁) 50.71±10.04 52.70±11.27 t=-0.230 0.042 BMI(kg/m2) 22.56(21.45~24.25) 22.84(21.88~24.24) Z=-1.572 0.116 WBC(×109/L) 3.93(3.02~5.55) 3.96(2.87~5.11) Z=-0.800 0.424 HGB(g/L) 127.00(108.00~142.00) 117.00(96.00~134.00) Z=-4.380 <0.001 PLT(×109/L) 83.00(55.00~116.00) 73.00(53.00~105.00) Z=-2.408 0.016 TBil(mmol/L) 31.80(21.50~53.50) 34.00(21.00~62.70) Z=-0.331 0.741 AST(U/L) 58.00(37.00~109.00) 54.00(38.00~100.00) Z=-0.795 0.427 ALT(U/L) 39.00(25.00~82.00) 39.000(26.00~66.00) Z=-0.711 0.477 TP(g/L) 63.63±8.94 61.11±8.29 t=3.615 0.364 Alb(g/L) 32.00(28.10~37.10) 28.80(25.30~33.30) Z=-5.999 <0.001 TG(mmol/L) 0.82(0.60~1.14) 0.72(0.52~1.01) Z=-2.831 0.005 CHOL(mmol/L) 3.38(2.72~3.96) 3.19(2.51~3.85) Z=-2.379 0.017 HDL(mmol/L) 0.95(0.61~1.24) 0.88(0.50~1.23) Z=-1.841 0.066 LDL(mmol/L) 1.90(1.45~2.30) 1.76(1.32~2.25) Z=-2.395 0.017 hs-CRP(mg/L) 3.46(1.24~10.10) 5.88(1.93~15.11) Z=-3.539 <0.001 IL-6(pg/mL) 12.61(8.72~23.60) 16.81(8.65~30.61) Z=-2.733 0.006 PT(s) 16.70(15.30~18.50) 17.50(15.70~19.70) Z=-3.374 0.001 CD4+(个/μL) 461.90(313.30~620.73) 359.70(263.16~517.81) Z=-4.902 <0.001 注:HIV,人类免疫缺陷病毒;TIPS,经颈静脉肝内门体静脉分流术;PSE,部分脾动脉栓塞术;NSBB,β受体阻滞剂;HE,肝性脑病;Child-Pugh分级,蔡尔德-皮尤分级;MELD,终末期肝病模型;BMI,体重指数;WBC,白细胞;HGB,血红蛋白;PLT,血小板;TBil,总胆红素;AST,天冬氨酸氨基转移酶;ALT,丙氨酸氨基转移酶;TP,总蛋白;Alb,白蛋白;TG,甘油三酯;CHOL,总胆固醇;HDL,高密度脂蛋白;LDL,低密度脂蛋白;hs-CRP,超敏C反应蛋白;IL-6,白细胞介素6;PT,凝血酶原时间;CD4+,CD4 T淋巴细胞计数。
表 3 PSM后肝硬化失代偿期患者再代偿影响因素的Cox单因素回归分析
Table 3. Univariate risk factor analysis of recompensation in decompensated liver cirrhosis patients after PSM using Cox regression
变量 HR 95%CI P值 变量 HR 95%CI P值 年龄(岁) 0.989 0.979~1.000 0.041 Child-Pugh分级 男性 1.305 1.028~1.658 0.032 A级 1.000 2型糖尿病史 0.921 0.675~1.257 0.604 B级 0.652 0.493~0.861 0.003 高血压病史 0.707 0.514~0.973 0.033 C级 0.494 0.361~0.676 <0.001 饮酒史 0.941 0.747~1.186 0.607 MELD评分 吸烟史 0.849 0.674~1.069 0.164 低危 1.000 HIV感染史 0.612 0.303~1.235 0.171 中危 0.480 0.352~0.655 <0.001 TIPS史 0.480 0.294~0.784 0.003 高危 0.457 0.345~0.590 <0.001 PSE史 0.738 0.514~1.059 0.099 BMI(kg/m2) 0.981 0.936~1.028 0.420 内镜治疗史 1.157 0.850~1.574 0.355 WBC(×109/L) 1.003 0.971~1.036 0.876 口服NSBB 0.760 0.541~1.068 0.113 HGB(g/L) 1.008 1.004~1.012 <0.001 HE 1.811 0.964~3.401 0.065 PLT(×109/L) 1.003 1.001~1.005 0.006 感染 1.423 1.116~1.815 0.004 TBil(mmol/L) 1.000 0.998~1.001 0.489 病因治疗 0.746 0.409~1.188 0.217 AST(U/L) 1.000 1.000~1.001 0.204 失代偿事件 0.792 0.530~1.183 0.254 ALT(U/L) 1.001 1.000~1.001 0.017 病因 TP(g/L) 1.023 1.009~1.036 0.001 乙型肝炎 1.000 Alb(g/L) 1.043 1.027~1.061 <0.001 丙型肝炎 3.702 2.767~4.951 <0.001 TG(mmol/L) 1.288 1.089~1.523 0.003 酒精性肝炎 3.545 2.329~5.394 <0.001 CHOL(mmol/L) 1.114 1.025~1.210 0.011 自身免疫性肝病 4.627 2.431~8.810 <0.001 HDL(mmol/L) 1.201 0.970~1.487 0.092 腹水分级 LDL(mmol/L) 1.150 1.018~1.300 0.025 无 1.000 hs-CRP(mg/L) 0.986 0.976~0.995 0.003 少量 0.508 0.369~0.689 <0.001 IL-6(pg/mL) 0.995 0.992~0.999 0.012 中量 0.507 0.355~0.724 <0.001 PT(s) 0.945 0.907~0.985 0.007 大量 0.295 0.183~0.478 <0.001 CD4+(个/μL) 1.001 1.001~1.002 <0.001 注:HIV,人类免疫缺陷病毒;TIPS,经颈静脉肝内门体静脉分流术;PSE,部分脾动脉栓塞术;NSBB,β受体阻滞剂;HE,肝性脑病;Child-Pugh分级,蔡尔德-皮尤分级;MELD,终末期肝病模型;BMI,体重指数;WBC,白细胞;HGB,血红蛋白;PLT,血小板;TBil,总胆红素;AST,天冬氨酸氨基转移酶;ALT,丙氨酸氨基转移酶;TP,总蛋白;Alb,白蛋白;TG,甘油三酯;CHOL,总胆固醇;HDL,高密度脂蛋白;LDL,低密度脂蛋白;hs-CRP,超敏C反应蛋白;IL-6,白细胞介素6;PT,凝血酶原时间;CD4+,CD4 T淋巴细胞计数;HR,风险比;CI,置信区间。
表 4 PSM后肝硬化失代偿期患者再代偿影响因素的Cox多因素回归分析
Table 4. Multivariate risk factor analysis of recompensation in decompensated liver cirrhosis patients after PSM using Cox regression
变量 HR 95%CI P值 男性 0.701 0.524~0.937 0.016 病因 乙型肝炎 1.000 丙型肝炎 0.288 0.131~0.634 0.002 酒精性肝炎 1.139 0.539~2.407 0.733 自身免疫性肝病 0.862 0.378~1.962 0.723 年龄(岁) 0.988 0.976~1.000 0.047 HGB(g/L) 1.006 1.001~1.011 0.017 CD4+(个/μL) 1.001 1.000~1.001 0.047 TIPS史 1.808 1.080~3.026 0.024 注:血红蛋白;CD4+,CD4 T淋巴细胞计数;TIPS,经颈静脉肝内门体分流术;HR,风险比;CI,置信区间。
-
[1] Chinese Society of Hepatology, Chinese Medical Association. Guidelines on the management of ascites in cirrhosis(2023 version)[J]. Chin J Hepatol, 2023, 31( 8): 813- 826. DOI: 10.3760/cma.j.cn501113-20230719-00011.中华医学会肝病学分会. 肝硬化腹水诊疗指南(2023年版)[J]. 中华肝脏病杂志, 2023, 31( 8): 813- 826. DOI: 10.3760/cma.j.cn501113-20230719-00011. [2] WANG Q, ZHAO H, DENG Y, et al. Validation of Baveno VII criteria for recompensation in entecavir-treated patients with hepatitis B-related decompensated cirrhosis[J]. J Hepatol, 2022, 77( 6): 1564- 1572. DOI: 10.1016/j.jhep.2022.07.037. [3] DENG Y, KANG HY, XIANG HL, et al. Durability and on-treatment predictors of recompensation in entecavir-treated patients with hepatitis B and decompensated cirrhosis[J]. JHEP Rep, 2024, 6( 7): 101091. DOI: 10.1016/j.jhepr.2024.101091. [4] RUAN JJ, WEN SF, WANG X, et al. Influencing factors for recompensation in patients with first-time decompensated hepatitis B cirrhosis[J]. J Clin Hepatol, 2022, 38( 8): 1796- 1800. DOI: 10.3969/j.issn.1001-5256.2022.08.015.阮佳佳, 温世飞, 王霞, 等. 首次失代偿期乙型肝炎肝硬化患者获得再代偿的影响因素分析[J]. 临床肝胆病杂志, 2022, 38( 8): 1796- 1800. DOI: 10.3969/j.issn.1001-5256.2022.08.015. [5] Chinese Society of Hepatology, Chinese Medical Association. Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of hepatitis C(2022 version)[J]. Chin J Infect Dis, 2023, 41( 1): 29- 46. DOI: 10.3760/cma.j.cn311365-20230217-00045.中华医学会肝病学分会, 中华医学会感染病学分会. 丙型肝炎防治指南(2022年版)[J]. 中华传染病杂志, 2023, 41( 1): 29- 46. DOI: 10.3760/cma.j.cn311365-20230217-00045. [6] Chinese Society of Hepatology, Chinese Medical Association, Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(version 2022)[J]. Infect Dis Info, 2023, 36( 1): 1- 17. DOI: 10.3969/j.issn.1007-8134.2023.01.01.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2022年版)[J]. 传染病信息, 2023, 36( 1): 1- 17. DOI: 10.3969/j.issn.1007-8134.2023.01.01. [7] Fatty Liver Expert Committee, Chinese Medical Doctor Association, National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association. Guidelines of prevention and treatment for alcoholic liver disease:a 2018 update[J]. J Clin Hepatol, 2018, 34( 5): 939- 946. DOI: 10.3969/j.issn.1001-5256.2018.05.006.中国医师协会脂肪性肝病专家委员会, 中华医学会肝病学分会脂肪肝和酒精性肝病学组. 酒精性肝病防治指南(2018年更新版)[J]. 临床肝胆病杂志, 2018, 34( 5): 939- 946. DOI: 10.3969/j.issn.1001-5256.2018.05.006. [8] Chinese Society of Hepatology, Chinese Medical Association. Guidelines on the diagnosis and management of autoimmune hepatitis(2021)[J]. J Clin Hepatol, 2022, 38( 1): 42- 49. DOI: 10.3969/j.issn.1001-5256.2022.01.008.中华医学会肝病学分会. 自身免疫性肝炎诊断和治疗指南(2021)[J]. 临床肝胆病杂志, 2022, 38( 1): 42- 49. DOI: 10.3969/j.issn.1001-5256.2022.01.008. [9] DAI EH, GUO XR, WANG JT, et al. Investigate of the etiology and prevention status of liver cirrhosis[J]. Natl Med J China, 2023, 103( 12): 913- 919. DOI: 10.3760/cma.j.cn112137-20221017-02164.戴二黑, 郭心如, 王继涛, 等. 肝硬化的病因及防治现状调查[J]. 中华医学杂志, 2023, 103( 12): 913- 919. DOI: 10.3760/cma.j.cn112137-20221017-02164. [10] HUI VW, WONG GL, WONG VW, et al. Baveno VII criteria for recompensation predict transplant-free survival in patients with hepatitis B-related decompensated cirrhosis[J]. JHEP Rep, 2023, 5( 9): 100814. DOI: 10.1016/j.jhepr.2023.100814. [11] ZHANG YH, LIU X, LI S, et al. Risk of HCC decreases in HBV-related patients with cirrhosis acquired recompensation: A retrospective study based on Baveno VII criteria[J]. Hepatol Commun, 2024, 8( 1): e0355. DOI: 10.1097/HC9.0000000000000355. [12] HE ZY, WANG BQ, WU XN, et al. Recompensation in treatment-naïve HBV-related decompensated cirrhosis: A 5-year multi-center observational study comparing patients with ascites and bleeding[J]. Hepatol Int, 2023, 17( 6): 1368- 1377. DOI: 10.1007/s12072-023-10579-w. [13] PREMKUMAR M, DHIMAN RK, DUSEJA A, et al. Recompensation of chronic hepatitis C-related decompensated cirrhosis following direct-acting antiviral therapy: Prospective cohort study from a hepatitis C virus elimination program[J]. Gastroenterology, 2024, 167( 7): 1429- 1445. DOI: 10.1053/j.gastro.2024.08.018. [14] SEMMLER G, LENS S, HIDALGO Á, et al. Incidence and clinical significance of recompensation after HCV cure[J]. Clin Gastroenterol Hepatol, 2025: S1542-S3565(25)00414- 8. DOI: 10.1016/j.cgh.2025.04.026. [15] XU DQ, MU H, ZHANG YY, et al. Influencing factors for recompensation in patients with decompensated hepatitis C cirrhosis[J]. J Clin Hepatol, 2025, 41( 2): 269- 276. DOI: 10.12449/JCH250212.许丹青, 木唤, 张映媛, 等. 丙型肝炎肝硬化失代偿患者再代偿的影响因素分析[J]. 临床肝胆病杂志, 2025, 41( 2): 269- 276. DOI: 10.12449/JCH250212. [16] HOFER BS, SIMBRUNNER B, HARTL L, et al. Hepatic recompensation according to Baveno VII criteria is linked to a significant survival benefit in decompensated alcohol-related cirrhosis[J]. Liver Int, 2023, 43( 10): 2220- 2231. DOI: 10.1111/liv.15676. [17] ZHANG P. Clinical characteristics and related factors research for recompensation in decompensated liver cirrhosis[D]. Chengdu: University of Electronic Science and Technology of China, 2024.张培. 失代偿期肝炎肝硬化再代偿临床特征及相关影响因素研究[D]. 成都: 电子科技大学, 2024. [18] HOFER BS, BURGHART L, HALILBASIC E, et al. Evaluation of potential hepatic recompensation criteria in patients with PBC and decompensated cirrhosis[J]. Aliment Pharmacol Ther, 2024, 59( 8): 962- 972. DOI: 10.1111/apt.17908. [19] DING SY, LU SY, LI JN, et al. Analysis of drinking patterns and risk factors for ascites in patients with alcoholic cirrhosis[J]. Trauma and Crit Medicine, 2025, 13( 5): 321- 325. DOI: 10.16048/j.issn.2095-5561.2025.05.01.丁思元, 卢盛言, 李佳宇, 等. 酒精性肝硬化患者饮酒模式与腹水发生的危险因素分析[J]. 创伤与急危重病医学, 2025, 13( 5): 321- 325. DOI: 10.16048/j.issn.2095-5561.2025.05.01. [20] XU XM, WANG HL, ZHAO WL, et al. Recompensation factors for patients with decompensated cirrhosis: A multicentre retrospective case-control study[J]. BMJ Open, 2021, 11( 6): e043083. DOI: 10.1136/bmjopen-2020-043083. [21] TONON M, GAGLIARDI R, POMPILI E, et al. Validation and expansion of Baveno VII recompensation criteria in patients with cirrhosis and curable liver disease[J]. J Hepatol, 2025, 83( 4): 888- 898. DOI: 10.1016/j.jhep.2025.04.018. [22] KUMAR A, MISHRA SR, SHARMA P, et al. Clinical, laboratory, and hemodynamic parameters in portal hypertensive gastropathy: A study of 254 cirrhotics[J]. J Clin Gastroenterol, 2010, 44( 4): 294- 300. DOI: 10.1097/MCG.0b013e3181b37ea1. [23] SINGH S, MANRAI M, PARVATHI VS, et al. Association of liver cirrhosis severity with Anemia: Does it matter?[J]. Ann Gastroenterol, 2020, 33( 3): 272- 276. DOI: 10.20524/aog.2020.0478. [24] ZHANG LL, DING XQ, YANG L. Effect of Anemia on prognosis in elderly patients with HBV-related decompensated cirrhosis[J]. J Navy Med, 2024, 45( 4): 408- 412. DOI: 10.3969/j.issn.1009-0754.2024.04.020.张玲玲, 丁小琴, 杨丽. 贫血对老年乙型肝炎病毒相关失代偿期肝硬化患者预后的影响[J]. 海军医学杂志, 2024, 45( 4): 408- 412. DOI: 10.3969/j.issn.1009-0754.2024.04.020. [25] RUDOLPH KL, CHANG S, MILLARD M, et al. Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery[J]. Science, 2000, 287( 5456): 1253- 1258. DOI: 10.1126/science.287.5456.1253. [26] WANG YX, WU CY, ZHOU JH, et al. Overexpression of estrogen receptor β inhibits cellular functions of human hepatic stellate cells and promotes the anti-fibrosis effect of calycosin via inhibiting STAT3 phosphorylation[J]. BMC Pharmacol Toxicol, 2022, 23( 1): 77. DOI: 10.1186/s40360-022-00617-y. [27] LI M, SU JT, WU SS, et al. Correlation among age, sex, and liver diseases-related mortality risk in patients with hepatitis B virus-related liver cirrhosis[J]. Chin J Hepatol, 2021, 29( 5): 403- 408. DOI: 10.3760/cma.j.cn501113-20201224-00676.李敏, 苏健婷, 武珊珊, 等. 乙型肝炎肝硬化患者年龄和性别与肝病相关死亡风险的关系[J]. 中华肝脏病杂志, 2021, 29( 5): 403- 408. DOI: 10.3760/cma.j.cn501113-20201224-00676. [28] CHANG XJ, LI YY, SUN C, et al. High-risk population of progressive hepatic fibrosis in chronic hepatitis B patients on antiviral therapy[J]. J Gastroenterol, 2023, 58( 5): 481- 493. DOI: 10.1007/s00535-023-01970-3. [29] ZHU NN, JIAO SN, ZHANG YS, et al. Analysis of influencing factors and predictive efficacy of recompensation in patients with decompensated hepatitis B cirrhosis[J]. Shandong Med J, 2025, 65( 6): 67- 72. DOI: 10.3969/j.issn.1002-266X.2025.06.014.朱宁宁, 焦淑宁, 张域爽, 等. 乙型肝炎肝硬化失代偿期患者再代偿的影响因素及其预测效能分析[J]. 山东医药, 2025, 65( 6): 67- 72. DOI: 10.3969/j.issn.1002-266X.2025.06.014. [30] LI X, ZHAO PP, WANG FB, et al. An analysis of the relationship between child- pugh score and cellular immune function in patients with hepatitis B cirrhosis[J]. Labeled Immunoass Clin Med, 2022, 29( 6): 932- 934. DOI: 10.11748/bjmy.issn.1006-1703.2022.06.008.李欣, 赵培培, 王富兵, 等. 乙肝后肝硬化患者Child-Pugh分级与细胞免疫功能相关性分析[J]. 标记免疫分析与临床, 2022, 29( 6): 932- 934. DOI: 10.11748/bjmy.issn.1006-1703.2022.06.008. [31] SÁNCHEZ J, GONZÁLEZ S, POYATOS P, et al. Recompensation after TIPS reduces the incidence of hepatocellular carcinoma and increases survival in patients with cirrhosis[J]. Liver Int, 2024, 44( 11): 3072- 3082. DOI: 10.1111/liv.16095. [32] BAI Y, LIU J, LEI Y, et al. Recompensation after transjugular intrahepatic portosystemicshunt reduces mortality risk: A long-term follow-up study[J]. Eur J Radiol, 2025, 190: 112212. DOI: 10.1016/j.ejrad. 2025.112212. -



 PDF下载 ( 1584 KB)
PDF下载 ( 1584 KB)

 下载:
下载: